Polonium-210 causes a very clear association with radiation. And this is not in vain, since it is extremely dangerous.
Discovery story
Its existence was predicted back in 1889 by Mendeleev, when he created his famous periodic table. In practice, this element number 84 was obtained nine years later by the efforts of the Curie spouses, who studied the phenomenon of radiation. Maria Skłodowska Curie tried to find out the cause of the strong radiation emanating from certain minerals, and therefore began working with several rock samples, processing them with all the methods available to her, dividing them into fractions and discarding unnecessary. As a result, she received a new substance, which became an analog of bismuth and the third open radioactive element after uranium and thorium.
Despite the successful results of the experiment, Maria was in no hurry to talk about her find. The spectral analysis carried out by a colleague of the Curie spouses also did not give reason to talk about the discovery of a new element. Nevertheless, in a report at a meeting of the Paris Academy of Sciences in July 1898, the couple reported on the alleged receipt of a substance exhibiting the properties of the metal and suggested that it be called polonium in honor of Poland, the birthplace of Mary. This was the first and only case in history when an element that has not yet been reliably identified has already been named. Well, the first sample appeared only in 1910.
Physical and chemical properties
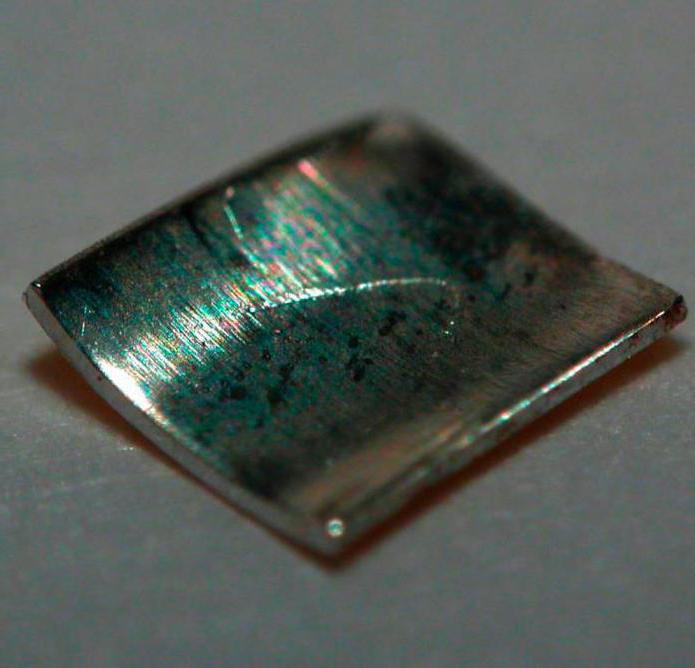
Polonium is a relatively soft silver-white metal. It is so radioactive that it glows in the dark and constantly heats up. At the same time, its melting temperature is slightly higher than that of tin - only 254 degrees Celsius. The metal oxidizes very quickly in air. At low temperatures, forms a monatomic simple cubic crystal lattice.
By its chemical properties, polonium is very close to its analogue - tellurium. In addition, the high level of radiation has a great influence on the nature of its compounds. So reactions involving polonium can be very spectacular and interesting, albeit quite dangerous in terms of health benefits.
Isotopes
In total, science currently knows 27 (according to other sources - 33) forms of polonium. None of them are stable, and they are all radioactive. The heaviest of the isotopes (with ordinal numbers from 210 to 218) are found in small quantities in nature, the rest can only be obtained by artificial means.
Radioactive polonium-210 is the most long-lived of its natural forms. It is contained in a small amount in radium-uranium ores and is formed due to a chain of reactions starting with U-238 and lasting about 4.5 billion years, if we talk about the half-life.
Getting
1 ton of uranium ore contains the polonium-210 isotope in an amount equal to about 100 micrograms. They can be distinguished in the processing of production waste, however, to obtain a more or less significant volume of the element would have to process a huge amount of material. A much simpler and more efficient method is fusion using neutron irradiation of natural bismuth in nuclear reactors.
As a result, after some more procedures polonium-210 is obtained. Isotopes 208 and 209 can also be obtained by irradiating bismuth or lead with accelerated beams of alpha particles, protons, or deuterons.
Radioactivity
Polonium-210, like other isotopes, is an alpha emitter. The heavier group also emits gamma rays. Despite the fact that the isotope 210 is the source of only alpha particles, it is quite dangerous, it can not be taken with your hands or even come close to a distance, because when it warms up, it goes into an aerosol state. Polonium ingestion with breathing or food is also extremely dangerous. That is why work with this substance takes place in special airtight boxes. Interestingly, this element was discovered in tobacco leaves about half a century ago. The decay period of polonium-210 compared with other isotopes is quite large, and therefore it can accumulate in the plant and subsequently harm the smoker's health even more. However, any attempts to extract this substance from tobacco were unsuccessful.
Danger
Since polonium-210 emits only alpha particles, observing certain precautions, you should not be afraid of working with it. The path length of these waves rarely exceeds ten centimeters, in addition, they usually can not penetrate the skin.
However, once inside the body, they cause him great harm. When it enters the bloodstream, it quickly spreads to all tissues - after a few minutes, its presence can be seen in all organs. First of all, it is present in the kidneys and liver, but in general, it is distributed fairly evenly, which can explain its high overall damaging effect.
The toxicity of polonium is so great that even small doses cause chronic radiation sickness and death after 6-11 months. The main ways of excretion from the body are through the kidneys and gastrointestinal tract. Dependence on the method of hit. The elimination half-life makes from 30 to 50 days.
Accidental polonium poisoning is completely impossible. In order to obtain a sufficient quantity of a substance, it is necessary to have access to a nuclear reactor and intentionally place an isotope to the victim. The complexity of the diagnosis also lies in the fact that only a few cases are known in history. The first victim is considered to be the daughter of the discoverers of polonium - Irene Joliot-Curie, who, during research, broke a capsule with a substance in the laboratory and died 10 years later. Two more cases are in the 21st century. The first of them is the sensational case of Litvinenko, who died in 2006, and the second is the death of Yasser Arafat, in whose things traces of a radioactive isotope were found. However, the final diagnosis has not been confirmed.
Decay
One of the most long-lived isotopes, along with 208 and 209, is polonium-210. The half-life (that is, the time during which the number of radioactive particles is halved) in the first two is 2.9 and 102 years, respectively, and the last 138 days and 9 hours. As for the remaining isotopes, their lifetime is calculated mainly in minutes and hours.
The combination of various properties of polonium-210 makes it the most convenient of a number for use in various fields of life. Being in a special metal shell, he can no longer harm health, but is able to give his energy for the benefit of mankind. So what is polonium 210 used for today?
Modern application
According to some reports, about 95% of polonium production is concentrated in Russia, with about 100 grams of the substance being synthesized per year, and almost all of it is exported to the United States.
There are several areas in which polonium-210 is used. First of all, these are spacecraft. With its compact size, it is indispensable as an excellent source of energy and heat. Although approximately every 5 months its efficiency is halved, heavier isotopes are much more expensive to produce.
In addition, polonium is completely indispensable in nuclear physics. It is widely used in studying the effect of alpha radiation on other substances.
Finally, another area of application is the manufacture of devices for removing static electricity for both industry and home use. It is even amazing how such a dangerous element can become almost a kitchen utensil, being enclosed in a reliable shell.