Many are interested in the question of what is the structure of polymers. The answer will be given in this article. The properties of the polymer (hereinafter referred to as P) are generally divided into several classes depending on the scale at which the property is determined, as well as on its physical basis. The most basic quality of these substances is the identity of its constituent monomers (M). The second set of properties, known as a microstructure, essentially denotes the location of these M in P on the scale of one C. These basic structural characteristics play a major role in determining the bulk physical properties of these substances, which show how P behaves as a macroscopic material. Nanoscale chemical properties describe how chains interact through various physical forces. On a macro scale, they show how basic P interacts with other chemicals and solvents.
Identity
The identity of the repeating units that make up P is its first and most important attribute. The nomenclature of these substances is usually based on the type of monomeric residues making up P. Polymers that contain only one type of repeating units are known as homo-P. At the same time, P containing two or more types of repeating units are known as copolymers. Terpolymers contain three types of repeating units.
Polystyrene, for example, consists only of styrene M residues and is therefore classified as homo-P. Ethylene vinyl acetate, on the other hand, contains more than one kind of repeating units and, therefore, is a copolymer. Some biological Ps consist of many different but structurally related monomeric residues; for example, polynucleotides, such as DNA, are composed of four types of nucleotide subunits.
A polymer molecule containing ionizable subunits is known as a polyelectrolyte or ionomer.
Microstructure
The polymer microstructure (sometimes called configuration) is associated with the physical arrangement of M residues along the main chain. These are elements of structure that require breaking the covalent bond in order to change. The structure has a strong influence on other properties of P. For example, two samples of natural rubber may show different durability, even if their molecules contain the same monomers.
The structure and properties of polymers
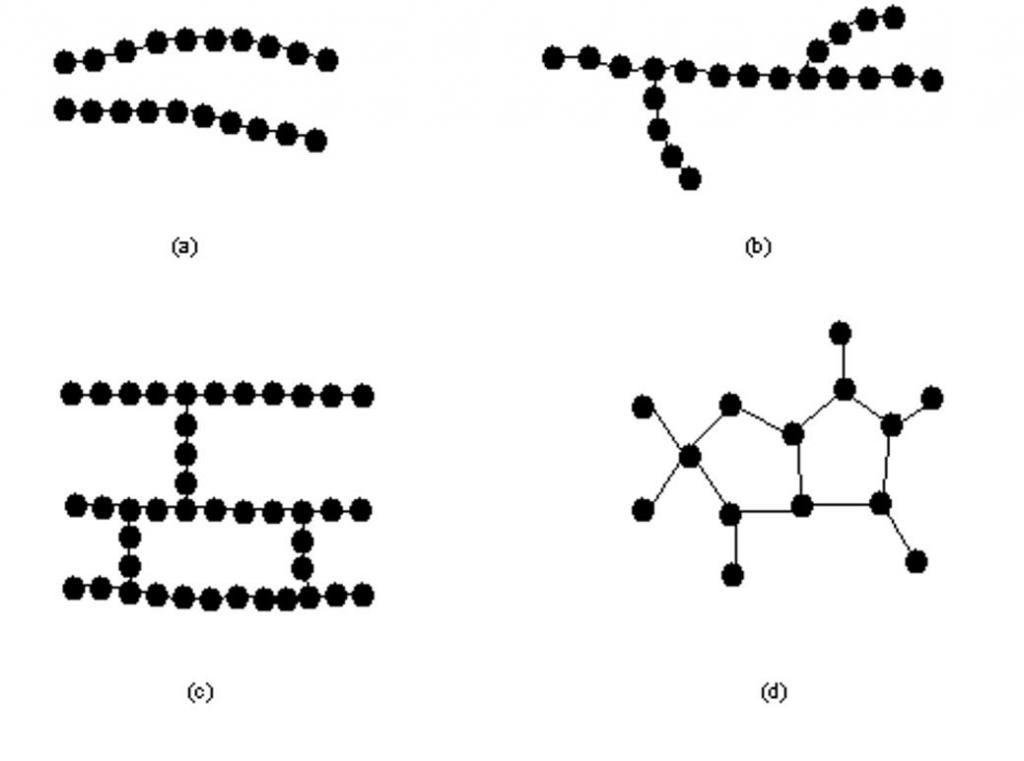
This point is extremely important to clarify. An important microstructural feature of the polymer structure is its architecture and shape, which are related to how branch points lead to a deviation from a simple linear chain. The branched molecule of this substance consists of a main chain with one or more side chains or branches of a substituent. Branched P types include star-shaped, comb-shaped P, brush P, dendronized, stair, and dendrimers. There are also two-dimensional polymers that consist of topologically flat repeating units. A variety of techniques can be used to synthesize P-material with various types of devices, for example, live polymerization.
Other qualities
The composition and structure of polymers in the science of them is related to how branching leads to a deviation from a strictly linear P-chain. Branching can occur randomly, or reactions can be designed to target specific architectures. This is an important microstructural feature. The polymer architecture affects many of its physical properties, including solution viscosity, melt, solubility in various compositions, glass transition temperature, and the size of individual P-coils in solution. This is important for studying the contained components and the structure of polymers.
Branching
Branches can form when the growing end of the polymer molecule is fixed either (a) back to itself, or (b) to another P-chain, and both, due to the removal of hydrogen, can create a growth zone for the middle chain.
The branching effect - chemical crosslinking - the formation of covalent bonds between chains. Crosslinking tends to increase Tg and increase strength and toughness. Among other uses, this process is used to strengthen rubbers in a process known as vulcanization, which is based on sulfur crosslinking. Car tires, for example, have high strength and a degree of crosslinking to reduce air leakage and increase their durability. The elastic, on the other hand, is not stitched, which allows peeling of the rubber and prevents damage to the paper. The polymerization of pure sulfur at higher temperatures also explains why it becomes more viscous at elevated temperatures in the molten state.
Grid
A polymer with a high degree of crosslinking is called a P-network. A sufficiently high ratio of crosslinking to chain (C) can lead to the formation of a so-called endless network or gel in which each such branch is connected to at least one other.
With the continuous development of living polymerization, the synthesis of these substances with a specific architecture is becoming easier. Such architectures as star-shaped, comb, brush, dendronized, dendrimers and ring polymers are possible. These chemical compounds with complex architecture can be synthesized either using specially selected starting compounds, or first by synthesizing linear chains that undergo further reactions to combine with each other. Knotted Ps consist of many intramolecular cyclization units in one P-chain (PC).
Branching
In general, the higher the degree of branching, the more compact the polymer chain. They also affect the entanglement of the chain, the ability to slide past each other, which, in turn, affects the bulk physical properties. Long chain strains can improve polymer strength, toughness, and glass transition temperature (Tg) due to an increase in the number of bonds in the compound. On the other hand, a random and short value of C can reduce the strength of the material due to a violation of the ability of the chains to interact with each other or crystallize, due to the structure of the polymer molecules.
An example of the effect of branching on physical properties can be found in polyethylene. High density polyethylene (HDPE) has a very low degree of branching, is relatively rigid and is used in the manufacture of, for example, body armor. On the other hand, low density polyethylene (LDPE) has a significant number of long and short branches, is relatively flexible, and is used in areas such as plastic films. The chemical structure of polymers contributes to just such their use.
Dendrimers
Dendrimers are a special case of a branched polymer, where each monomer unit is also a branch point. This tends to reduce intermolecular chain entanglement and crystallization. A related architecture, the dendritic polymer, is not ideally branched, but has similar properties to dendrimers due to their high degree of branching.
The degree of formation of the complexity of the structure that occurs during polymerization may depend on the functionality of the monomers used. For example, in the case of free radical polymerization of styrene, the addition of divinylbenzene, which has a functionality of 2, will lead to the formation of branched P.
Engineering Polymers
Engineering polymers include natural materials such as rubber, synthetic materials, plastics and elastomers. They are very useful raw materials, because their structures can be changed and adapted for the production of materials:
- with a range of mechanical properties;
- in a wide range of colors;
- with various transparency properties.
The molecular structure of polymers
A polymer consists of many simple molecules that repeat structural units called monomers (M). One molecule of this substance may consist of amounts from hundreds to a million M and have a linear, branched or mesh structure. Covalent bonds hold the atoms together, and the secondary bonds then hold the groups of polymer chains together, forming a polymaterial. Copolymers are types of this substance, consisting of two or more different types of M.
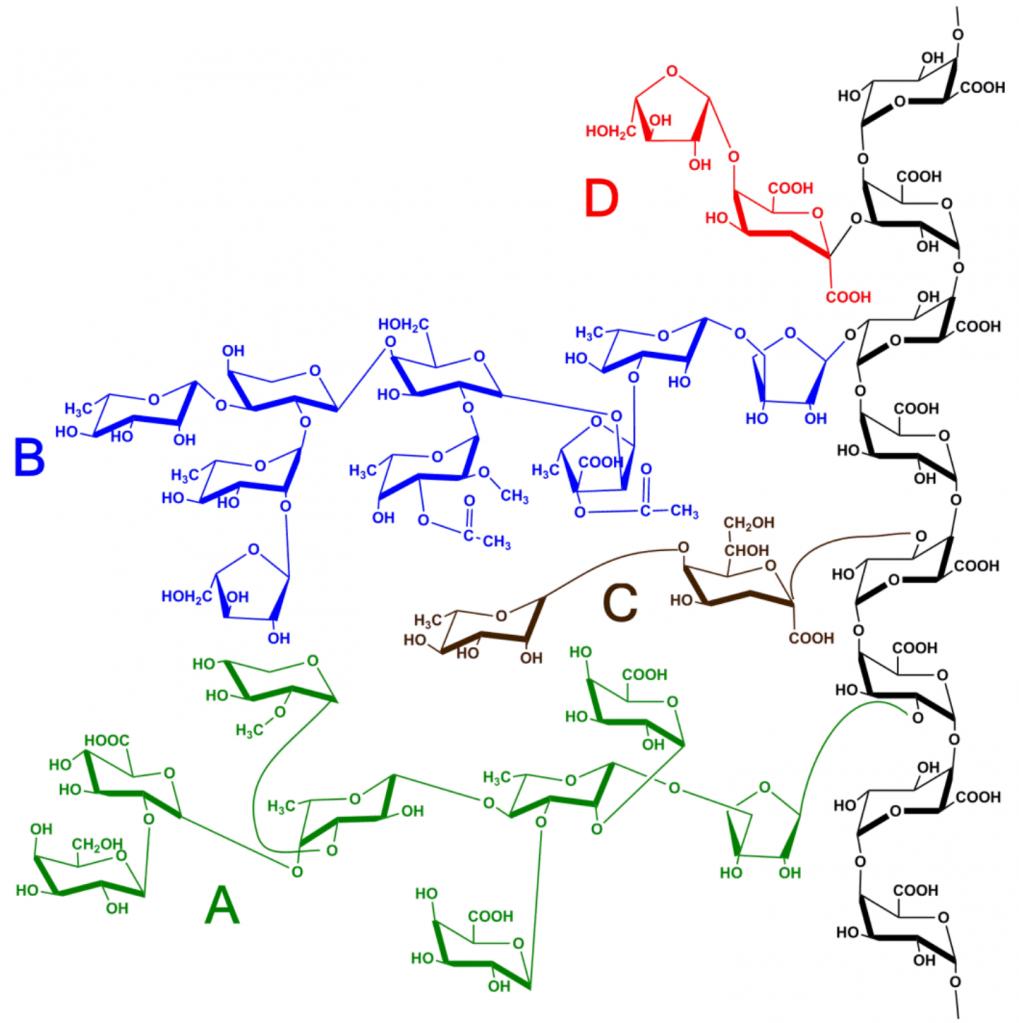
A polymer is an organic material, and the basis of any such type of substance is a chain of carbon atoms. The carbon atom has four electrons in the outer shell. Each of these valence electrons can form a covalent bond with another carbon atom or with a foreign atom. The key to understanding the polymer structure is that two carbon atoms can have up to three common bonds and still bind to other atoms. Elements that are most often found in this chemical compound, and their valence numbers: H, F, Cl, Bf and I with 1 valence electron; O and S with 2 valence electrons; n with 3 valence electrons; and C and Si with 4 valence electrons.
Polyethylene Example
The ability of molecules to form long chains is vital for polymer production. Consider a polyethylene material that is made from gaseous ethane, C2H6. Ethane gas has two carbon atoms in a chain, and each of them has two valence electrons with the other. If two ethane molecules are joined together, one of the carbon bonds in each molecule can be broken and the two molecules can be connected by a carbon-carbon bond. After two meters are connected, two more free valence electrons remain at each end of the chain to connect other meters or P-chains. The process is able to continue connecting more meters and polymers together until it is stopped by the addition of another chemical (terminator) that fills the available bond at each end of the molecule. This is called a linear polymer and is the building block for thermoplastic compounds.
The polymer chain is often shown in two dimensions, but it should be noted that they have a three-dimensional structure of polymers. Each bond is at an angle of 109 ° to the next, and therefore the carbon skeleton passes through space, like a TinkerToys twisted chain. When a voltage is applied, these chains are stretched, and the elongation P can be thousands of times greater than in crystalline structures. These are the structural features of polymers.