One of the tasks of chemistry is to study the structure of matter, including elucidating the mechanism of formation of various compounds from simple substances formed by atoms of one chemical element. Features of the interaction of atoms, more precisely, their oppositely charged components — electron shells and nuclei — are described as different types of chemical bonds. So, substances with a molecular structure are formed through a covalent bond, to describe which in 1931 the American chemist L. Pauling proposed a model of hybridization of atomic orbitals.
The concept of covalent bond
In those cases when a pair of valence electron clouds common to two atoms is formed during the interaction, they speak of covalent bonding. As a result of its occurrence, the smallest particle of a simple or complex substance is formed - a molecule.
One of the features of covalent bonding is its directivity - a consequence of the complex shape of the electronic orbitals p, d and f, which, without spherical symmetry, have a certain spatial orientation. Another important feature of this type of chemical bond is saturation, due to the limited number of external - valence - clouds in the atom. That is why the existence of a molecule, for example, H 2 O, is possible, but H 5 O is not.
Types of covalent bond
The formation of common electron pairs can occur in various ways. An important role in the mechanism of covalent bond formation is played by the nature of cloud overlapping and the spatial symmetry of the resulting cloud. According to this criterion, L. Pauling suggested distinguishing the following types:
- The sigma-bond (σ) is characterized by the greatest degree of overlap along the axis passing through the atomic nuclei. Here the cloud density will be maximum.
- The pi bond (π) is formed during lateral overlap, and the electron cloud, respectively, has the highest density outside the axis connecting the core.
These spatial characteristics are of great importance insofar as they correlate with the energy parameters of the covalent bond.
Features of polyatomic molecules
The concept of hybridization of atomic orbitals was introduced by Pauling to explain one of the features of covalent bonds in polyatomic molecules. It is known that the bonds formed by the central atom in such molecules turn out to be the same in spatial and energy characteristics. This happens regardless of which orbitals (s, p or d) are involved in the formation of a common electron pair.
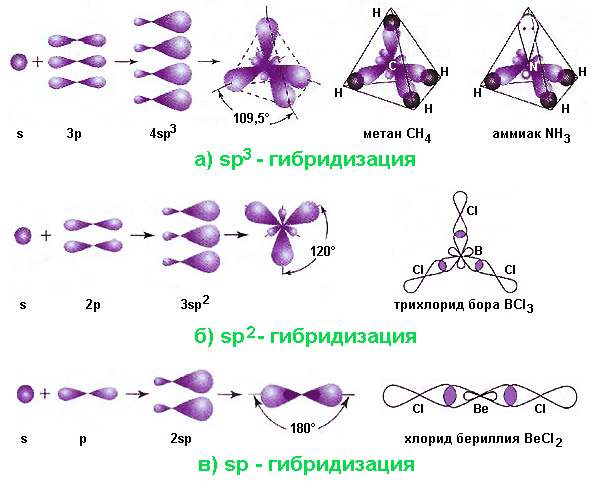
A very convenient and illustrative example to illustrate this phenomenon is the carbon atom. Upon entering into a chemical bond, an atom in an excited state has 4 valence orbitals: 2s, 2p x , 2p y and 2p z . The last three differ from the 2s orbital in energy and shape. Nevertheless, in a molecule, for example, methane CH 4, all four bonds are completely equivalent and have valence angles of 109.5 ° (while p-orbitals are located at 90 ° angles). In other carbon compounds, valence angles of 120 ° and 180 ° are found; in molecules containing nitrogen (ammonia NH 3 ) and oxygen (water H 2 O), these angles are 107.5 ° and 104.5 °. The occurrence of such valence angles also required explanation.
The essence of the phenomenon
The idea of hybridization is to form averaged orbitals by overlapping different types of electron clouds with close energy values - s, p, sometimes d. The number of resulting - hybrid - orbitals corresponds to the number of overlapping clouds. Since the orbital is a wave function that determines the probability of an electron being at a particular point in an atom, a hybrid orbital is an overlap of wave functions that occurs as a result of electronic transitions when an atom is excited. It leads to the appearance of equivalent wave functions that differ only in directivity.
Hybrid orbitals are equivalent in energy and have the same shape as a volume eight having strong asymmetry relative to the nucleus. Less energy is spent on hybridization than is released when a strong covalent bond is formed with hybrid orbitals; therefore, such a process is energetically favorable, that is, most likely.
The main types of hybridization of orbitals and the geometry of molecules
Various variants of overlapping (mixing) of external electron clouds in an atom are possible. The most common types of overlapping orbitals are:
- Sp 3 Hybridization. This option is realized when one s- and three p-orbitals are superimposed. Its result is four hybrid orbitals whose axes are directed for any pair at angles of 109.5 °, corresponding to the minimum mutual repulsion of electrons. When these orbitals enter into σ bonds with other atoms, a tetrahedral molecule is formed, for example, methane, ethane C 2 H 6 (a combination of two tetrahedra), ammonia, water. In the ammonia molecule, one, and in the water molecule, two of the vertices of the tetrahedron are occupied by lone electron pairs, which leads to a decrease in the valence angle.
- Sp 2 hybridization occurs when a combination of one s- and two p-orbitals. In this case, the three hybrid orbitals are located at angles of 120 ° in the same plane. A similar triangular shape are, for example, molecules of boron trichloride BCl 3 , which is used in various technologies. Another example, an ethylene molecule, is formed due to an additional π-bond between carbon atoms, in which the p-orbitals are non-hybrid and are oriented perpendicular to the plane formed by two triangles.
- Sp hybridization occurs when one s- and one p-orbital are mixed. Two hybrid clouds are located at an angle of 180 °, and the molecule has a linear configuration. Examples are beryllium chloride molecules BeCl 2 or acetylene C 2 H 2 (in the latter two non-hybrid carbon p-orbitals form additional π-bonds).
There are more complex variants of hybridization of atomic orbitals: sp 3 d, sp 3 d 2 and others.
The role of the hybridization model
Pauling's concept gives a good qualitative description of the structure of molecules. It is convenient and intuitive; it successfully explains some of the features of covalent compounds, such as the value of the valence angles or the alignment of the chemical bond length. However, the quantitative side of the model cannot be considered satisfactory, since it does not allow one to make many important predictions regarding the physical effects associated with structural features of the molecules, for example, molecular photoelectron spectra. The author of the concept of hybridization in the early 1950s noted its shortcomings.
Nevertheless, in the formation of modern ideas about the structure of matter, the hybridization model of atomic orbitals played a large role. Based on it, more adequate concepts were developed, for example, the theory of repulsion of electron pairs. Therefore, of course, the hybridization model was an important stage in the development of theoretical chemistry, and when describing some aspects of the electronic structure of molecules, it is quite applicable at the present time.