Our article will be devoted to the study of the properties of substances, which are the basis of the phenomenon of life on Earth. Protein molecules are present in non-cellular forms - viruses, are part of the cytoplasm and organoids of prokaryotic and nuclear cells. Along with nucleic acids, they form a substance of heredity - chromatin and form the main components of the nucleus - chromosomes. Signal, construction, catalytic, protective, energy - this is a list of biological functions that proteins perform. Physico-chemical properties of proteins are their ability to solubility, precipitation, salting out. In addition, they are able to denature and are amphoteric compounds by their chemical nature. We will study these properties of proteins further.
Types of Protein Monomers
20 types of α-amino acids are structural units of a protein. In addition to the hydrocarbon radical, they contain an NH 2 amino group and a COOH carboxyl group. Functional groups determine the acidic and basic properties of protein monomers. Therefore, in organic chemistry, compounds of this class are called amphoteric substances. Hydrogen ions of the carboxyl group within the molecule can be cleaved and bound to amino groups. The result is an internal salt. If several carboxyl groups are present in the molecule, the compound will be acidic, such as glutamic or aspartic acid. If amino groups predominate, amino acids are the main ones (histidine, lysine, arginine). With an equal number of functional groups, the peptide solution has a neutral reaction. It has been established that the presence of all three types of amino acids affects what features proteins will have. Physico-chemical properties of proteins: solubility, hydrogen index, macromolecule charge, are determined by the ratio of acidic and basic amino acids.
What factors affect the solubility of peptides
Let us find out all the necessary criteria on which the hydration or solvation of protein macromolecules depends. These are: spatial configuration and molecular weight, determined by the number of amino acid residues. The ratio of polar and nonpolar parts — radicals located on the surface of the protein in the tertiary structure and the total charge of the polypeptide macromolecule — is also taken into account. All of the above properties directly affect the solubility of the protein. Let's consider them in more detail.
Globules and their ability to hydrate
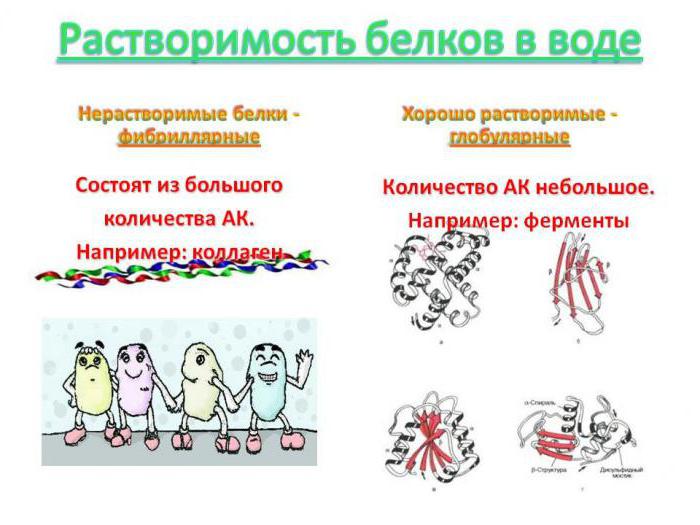
If the external structure of the peptide has a spherical shape, then it is customary to talk about its globular structure. It is stabilized by hydrogen and hydrophobic bonds, as well as by the forces of electrostatic attraction of oppositely charged parts of the macromolecule. For example, hemoglobin, which carries oxygen molecules through the blood, in quaternary form consists of four fragments of myoglobin combined by heme. Blood proteins such as albumin, α - and ϒ-globulins easily interact with substances in the blood plasma. Insulin is another globular peptide that regulates the level of glucose in the blood of mammals and humans. The hydrophobic parts of such peptide complexes are located in the middle of the compact structure, while the hydrophilic parts are located on its surface. This ensures that they retain their native properties in the body’s fluid and combines them into a group of water-soluble proteins. The exception is globular proteins, which form the mosaic structure of the membranes of human and animal cells. They are associated with glycolipids and are insoluble in the intercellular fluid, which ensures that they fulfill a barrier role in the cell.
Fibrillar peptides
Collagen and elastin, which are part of the dermis and determine its firmness and elasticity, have a threadlike structure. They are able to stretch, changing their spatial configuration. Fibroin is a protein of natural silk, produced by silkworm larvae. It contains short structural fibers consisting of amino acids with a small mass and a molecule length. These are, first of all, serine, alanine and glycine. Its polypeptide chains are oriented in space in the vertical and horizontal direction. The substance belongs to structural polypeptides and has a layered form. Unlike globular polypeptides, the solubility of a protein consisting of fibrils is very small, since the hydrophobic radicals of its amino acids lie on the surface of the macromolecule and repel the polar particles of the solvent.
Keratins and structural features
Considering a group of structural proteins of a fibrillar form, such as fibroin and collagen, it is necessary to dwell on another group of peptides, widely distributed in nature, - keratins. They serve as the basis for such parts of the human body and animals as hair, nails, feathers, wool, hooves and claws. What is keratin from the point of view of its biochemical structure? It has been established that there are two types of peptides. The first has the appearance of a spiral secondary structure (α-keratin) and is the basis of hair. The other is represented by more rigid layered fibrils - this is β-keratin. It can be found in solid parts of animals: hooves, bird beaks, reptile scales, claws of carnivorous mammals and birds. What is keratin, based on the fact that its amino acids, for example, such as valine, phenylalanine, isoleucine, contain a large number of hydrophobic radicals? It is a protein insoluble in water and other polar solvents that performs protective and structural functions.
The effect of pH on the charge of the protein polymer
Earlier, we mentioned that the functional groups of protein monomers - amino acids, determine their properties. We add now that the charge of the polymer depends on them. Ionogenic radicals - carboxyl groups of glutamic and aspartic acid and amino groups of arginine and histidine affect the total charge of the polymer. They also behave differently in an acidic, neutral or alkaline solution. The solubility of the protein depends on these factors. So, at pH <7, the solution contains an excessive concentration of hydrogen protons, which inhibit the cleavage of the carboxyl, therefore the total positive charge on the protein molecule increases.
The accumulation of cations in the protein also increases in the case of a neutral solution environment and with an excess of arginine, histidine, and lysine monomers. In an alkaline environment, an increase in the negative charge of the polypeptide molecule occurs, since an excess of hydrogen ions is spent on the formation of water molecules by binding hydroxyl groups.
Protein Solubility Factors
Imagine a situation in which the number of positive and negative charges on a protein helix is the same. The pH of the medium in this case is called the isoelectric point. The total charge of the peptide macromolecule itself becomes zero, and its solubility in water or another polar solvent will be minimal. The theory of electrolytic dissociation states that the solubility of a substance in a polar solvent consisting of dipoles will be the higher, the more polarized the particles of the dissolved compound. They also explain the factors that determine the solubility of proteins: their isoelectric point and the dependence of the hydration or solvation of the peptide on the total charge of its macromolecule. Most polymers of this class contain an excess of –COO– groups and have slightly acidic properties. An exception will be the previously mentioned membrane proteins and peptides that are part of the nuclear substance of heredity - chromatin. The latter are called histones and have pronounced basic properties due to the presence of a large number of amino groups in the polymer chain.
The behavior of proteins in an electric field
For practical purposes, it often becomes necessary to separate, for example, blood proteins into fractions or individual macromolecules. To do this, you can use the ability of charged polymer molecules to move at a certain speed to the electrodes in an electric field. A solution containing peptides of different masses and charges is placed on a carrier: paper or a special gel. By passing electrical impulses, for example, through a portion of blood plasma, up to 18 fractions of individual proteins are obtained. Among them: all types of globulins, as well as albumin, which is not only the most important component (it accounts for up to 60% of the mass of blood plasma peptides), but also plays a central role in the processes of osmosis and blood circulation.
How does salt concentration affect protein solubility?
The ability of peptides to form not only gels, foams and emulsions, but also solutions is an important property that reflects their physicochemical characteristics. For example, previously studied albumin in the endosperm of cereal seeds, milk and blood serum quickly form aqueous solutions with a concentration of neutral salts, for example, such as sodium chloride, in the range from 3 to 10 percent. Using the same albumin as an example, we can determine the dependence of protein solubility on salt concentration. They dissolve well in an unsaturated solution of ammonium sulfate, and in a supersaturated solution they are reversibly precipitated and, with a further decrease in salt concentration, restore their hydration shell by adding a portion of water.
Salting out
The chemical reactions of peptides described above with solutions of salts formed by strong acid and alkali are called salting out. It is based on the mechanism of interaction of charged functional groups of a protein with salt ions - metal cations and anions of acid residues. It ends with a loss of charge on the peptide molecule, a decrease in its water shell, and the adhesion of protein particles. As a result, they are precipitated, which we will dwell on.
Precipitation and denaturation
Acetone and ethyl alcohol destroy the water shell surrounding the protein in the tertiary structure. However, this is not accompanied by the neutralization of the total charge on it. Such a process is called precipitation, the solubility of the protein decreases sharply, but does not end with denaturation.
Molecules of peptides in their native state are very sensitive to many environmental parameters, for example, temperature and concentration of chemical compounds: salts, acids or alkalis. The enhancement of the action of both of these factors at the isoelectric point leads to the complete destruction of stabilizing intramolecular (disulfide bridges, peptide bonds), covalent and hydrogen bonds in the polypeptide. Globular peptides denature especially rapidly under such conditions, while completely losing their physicochemical and biological properties.