The group of lipids - important organic components of plant and animal cells - in addition to waxes and steroids, also includes fats. They are not only the most common, but also the most significant energy suppliers for all vital functions of the body: the synthesis of plastic substances, growth, reproduction. What is fat, what components it consists of, what properties and functions are characteristic for it - you will receive answers to these and many other questions in our article.
Features of physical properties
The ability to dissolve in organic solvents and antagonistic attitude to water is the hallmark of lipids. All of them are lighter than water and oily to the touch. Leather, paper, and other fibrous or porous materials quickly absorb their excess. From experience it is known that stains of fat on clothing can be removed using gasoline, carbon tetrachloride, acetone or carbon disulfide. Esters of higher carboxylic acids and glycerol alcohol can have two states of aggregation: liquid or solid. Both vegetable and animal fats, the properties and application of which we are studying, are esters, the structural formula of which is determined by what carboxylic acids are included in their composition.
Organic acids
This is a large group of substances containing carboxyl groups linked by covalent bonds with hydrocarbon radicals. If they have pi bonds in the carbon skeleton, i.e. are unsaturated, then the compound will be liquid. For example, olive, linseed or sunflower oil contains oleic and linolenic acids with unsaturated types of chemical bonds. The exception here is coconut oil, which refers to vegetable lipids, but it is not liquid, but solid. Edible animal fats have a solid consistency. This is pork or beef fat, as well as butter and various varieties of spread. They contain saturated carboxylic acids - stearic and palmitic. Interestingly, natural lipids are not a separate compound, but a mixture of various glycerides - glycerol esters and high molecular weight carboxylic acids.
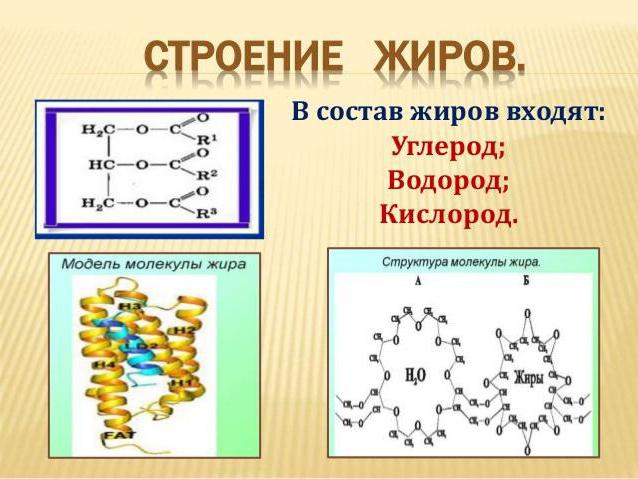
Molecule structure
For a person far from the theoretical foundations of chemistry and not familiar with the teachings of A. M. Butlerov, the structural formula of even the simplest lipid will seem cumbersome and obscure. In order not to complicate the topic, let’s say the following: the mandatory presence in the molecule of triatomic alcohol of glycerol and residues of organic acids with at least 15 carbon atoms in the carbon skeleton is what the fat of vegetable or animal origin consists of. And it does not matter whether the lipid is natural or whether it is obtained synthetically. The main thing is that all of them, without exception, belong to the class of esters and are formed as a result of the esterification reaction. Another interesting detail.
Until the beginning of the 19th century, there was an opinion in science that organic substances: proteins, carbohydrates and lipids - can only be obtained from living organisms. The French chemist M. Berthelot in 1854 synthesized fat in his laboratory from glycerol and fatty acids. By this he refuted erroneous ideas about the special exceptional nature of organic compounds and the impossibility of obtaining them artificially.
Esterification reaction
What is industrial fat made up of? Its composition depends on which carboxylic acids reacted with glycerol. Recall that if there are unsaturated acids in the composition of a triglyceride, it will be liquid, i.e., oil, and saturated carboxylic acids enter solid types of lipids. The esterification process is carried out in the presence of strong inorganic acids - sulfuric or chloride, the resulting products are quickly removed from the reaction sphere to prevent hydrolysis of the resulting fat. The initial reagents themselves are always taken in excess, this also increases its practical yield.
Types and functions of fats
So, the main aggregate states of these compounds are liquid or solid phases. Oils contain unsaturated carboxylic acids with one or more double bonds. For example, oleic acid has one pi-bond and is monounsaturated, found in olive or canola oil and is part of peanuts, avocados and olives. Fish oil, mussels, walnuts, as well as sunflower oil are rich in polyunsaturated acids: arachidonic, linoleic and linolenic. The biological functions of fats are not as diverse as, for example, in proteins, but all of them are vital. These are: protection, energy supply, excretory and thermal insulation properties. They are inherent in all substances of this class and do not depend on what the fat consists of, nor on what organism it is in. Lipids secreted by the sebaceous glands lubricate the skin and protect it from dryness. They do not allow the penetration of excess water into the skin, which provides protection from edema. The substances that make up the fatty body of insects, for example various species of beetles, accumulate toxic metabolic products, thereby performing an excretory function.

The nutritional value
Lipids can be distinguished by such a criterion as the speed of cleavage and the qualitative composition of the hydrolyzate. This indicator is necessarily taken into account when compiling various kinds of diets, in baby food, in organizing the diet of people suffering from chronic cardiovascular diseases and diseases of the gastrointestinal tract. What fats are good for the body? It is known that a diet rich in lipids containing Omega-3 and Omega-6 fatty acids helps to maintain normal cholesterol levels and favorably affects vascular permeability. For this, human food should contain linseed and olive oil, fish oil.
An excess of solid types of lipids in foods: lard, butter and margarine leads to the formation of cholesterol plaques on the walls of arteries that impede blood circulation and cause blood clots in them. This inevitably leads to an increased risk of coronary heart disease, often resulting in a heart attack. To avoid such problems, the use of fats should be subject to the following rule: in the diet of a person who is responsible for their health, the presence of products containing unsaturated fatty acids is necessary .
Hydrogenation: what is it?
Since we mentioned margarine, now is the time to find out how it is received. This is a solid artificially synthesized fat, the raw material for which are inexpensive varieties of edible vegetable oils. To transfer them to a solid state, a chemical reaction is carried out, saturating the oil with hydrogen, the atoms of which are attached at the sites of the breaking of double bonds in the residues of unsaturated acids. The process requires increased pressure, heating and the presence of powdered nickel as a catalyst. The resulting hydrogenation product, solid fat, is called salomas and is used as raw material in the production of glycerin, soap or stearin.
What is fat called margarine made of? This is a food product containing, in addition to salomas, animal lipids, milk, salt, sugar, vitamins, food colors and flavors. Such fat is often called light oil or spread, it is much cheaper than butter and less caloric, which allows it to be used in diet food.
Hydrolysis is the main chemical property
Previously, we found that fats consist of glycerol and fatty acids, which are the starting reagents in the esterification reaction. In the presence of water and under the influence of digestive enzymes in various sections of the gastrointestinal tract, they are split, which is accompanied by the release of the largest amount of energy compared to other organic compounds - proteins and carbohydrates. From one gram of fat with its complete oxidation, 38.9 kJ of energy can be obtained. This is two times more than with hydrolysis of glucose. Therefore, to the question of what fat is, the answer may be the following statement: this is the most important organic substance that provides cells with the energy necessary for their life. Moreover, the breakdown of lipids is accompanied by the release of a large number of H 2 O molecules.
Lipids as a hidden reservoir of water
Adapting to various abiotic conditions, living things strive to provide themselves with the compounds necessary for their vital functions, the main role among which is water. These are the inhabitants of the steppes and deserts: camels, jerboas, ground squirrels, field mice, etc. In addition, animals that carry winter or summer hibernation: brown bear, sand gopher, many species of shrews and insects, receive the necessary water also as a result of splitting reserves in the subcutaneous tissue or fat body. There are some types of insects, for example, clothes moth, which do not need external sources of water at all, but extract it from the reactions of dissimilation of organic substances.
Lipids as a building material of cells
The most important components of living systems are biological membranes. Due to the liquid-mosaic structure, they have unique functions: selective permeability, signaling and protective properties. The composition of all cell membranes includes lipids, about 30% of which are connected to protein globules, and the rest are in the liquid phase. We remember what substances fats consist of - these are glycerol and residues of organic acids, the molecules of which are arranged in a double layer. Hydrophilic components are oriented to the outer and inner parts of the membrane, and water-insoluble sections are turned in its middle. Most cellular organelles, such as the nucleus, chloroplasts, mitochondria, Golgi apparatus, endoplasmic reticulum, and lysosomes, have a membrane structure. All of them contain fat molecules, which are the building blocks of the cell.
Protective properties
Fats in the human body and other mammalian animals are part of subcutaneous fat. They can serve as armor that reliably covers vital organs, such as the kidneys, from mechanical shocks during movement, as well as from bumps and injuries. If the living conditions of the organism are extreme, for example, it is in ice water for a long time, then the fat reserves save it from hypothermia. In seals, walruses and fur seals, the lipid layer can be 15–20 cm, and in the largest animal in the world, the blue whale, it is more than half a meter thick! Therefore, the question of what fat can be answered in the following way: it is the main heat-insulating material of homeothermic organisms, i.e., those who maintain a constant temperature of their body.
What happens to fats in our body?
Foods rich in lipids are partially broken down in the stomach by the action of enzymes secreted by its mucous membrane. But their main hydrolysis occurs in the duodenum under the action of lipase, which is part of pancreatic juice. An important role belongs to the bile produced by the liver. It, like a crusher, breaks up lipid macromolecules into smaller parts - emulsifies them. This contributes to a better and faster hydrolysis process leading to the formation of glycerol and fatty acids. The hydrolyzate is absorbed by the villi of the small intestine first into the blindly closed capillaries of the lymphatic system, and already from them enters the blood. From glycerol and carboxylic acids, cells synthesize fats specific for a given organism, some of which can be deposited in subcutaneous fat and omentum - a kind of fat depot. During periods of prolonged starvation, with heavy physical exertion or stress, the body spends these reserves to get energy.
Lipid and carbohydrate metabolism
Both groups of organic compounds: sugars and fats, cells can be used as energy material. Excess glucose hepatocytes are converted into the form of a polymer - animal glycogen starch. The products of lipid hydrolysis also enter the liver, where they turn into the same glycogen. Excess carbohydrates that enter the gastrointestinal tract with improper nutrition, in turn, transforms into fat, and a person quickly gains weight. These facts serve as evidence of the relationship between fat and carbohydrate metabolism, confirming the important role of lipids in our body.