Aldehydes are called organic substances related to carbonyl compounds containing a functional group —COH, which is called a carbonyl group.
Depending on the nature of the hydrocarbon skeleton, aldehyde molecules are extreme, unsaturated and aromatic. Their molecules may also include halogen atoms or additional functional groups. The general formula for saturated aldehydes is C n H 2 n O. In accordance with the IUPAC nomenclature, their names end with the suffix –al.
Oxidation of aldehydes is important in industry, as they are quite readily converted to carboxylic acids. In this case, oxidizing agents can be copper hydroxide, silver oxide or even atmospheric oxygen.
The structure of the carbonyl group
The electronic structure of the double bond in the C = O group is characterized by the formation of one σ-bond and another π-bond. Atom C is in a state of sp 2 hybridization, a molecule of a flat structure with valence angles between bonds of about 120 0 . The difference between the double bond in this functional group lies in the fact that it is located between the carbon atom and the highly electronegative oxygen atom. As a result, the electrons are attracted to the O atom, which means that this bond is very polarized.
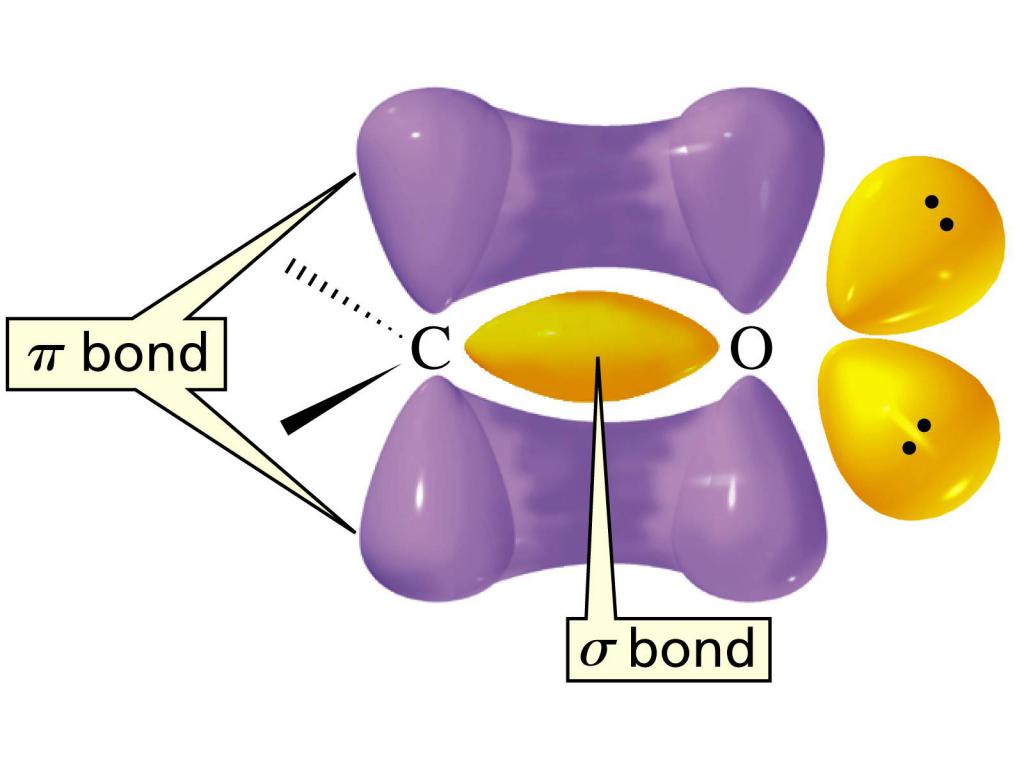
The content in the aldehyde group of such a polarized double bond can be called the main reason for the high reactivity of aldehydes. For aldehydes, the reactions of addition of atoms or their groups at the C = O bond are most characteristic. And nucleophilic addition reactions proceed most easily. Aldehydes are also characterized by reactions involving H atoms from the functional group of aldehydes. Due to the electron-withdrawing effect of the C = O group, an increase in the polarity of the bond occurs. This in turn is the reason for the relatively mild oxidation of aldehydes.
Individual aldehydes
Formaldehyde (formic aldehyde or methanal) CH 2 O is a gaseous substance with a very pungent odor, which is usually obtained by passing a mixture of methanol vapor with air through a red-hot grid of copper or silver mesh. Its 40% aqueous solution is called formalin. Formaldehyde easily reacts, many of which underlie the industrial synthesis of a number of important substances. It is also used to produce isoprene rubber, pentaerythritol, many medicinal substances, various dyes, for tanning the skin, as a disinfectant and deodorizing agent. Formaldehyde is quite toxic; its MPC in air is 0.001 mg / L.
Acetaldehyde (acetic aldehyde, ethanal) 3 is a colorless liquid with a suffocating odor, which, when diluted with water, acquires a fruity aroma. Acetaldehyde has all the basic properties of aldehydes. The oxidation of acetic aldehyde produces huge volumes of acetic acid and acetic anhydride, a variety of pharmaceuticals.
Acrolein (propenal) CH 2 = CH-SON, the simplest unsaturated aldehyde is a colorless volatile liquid. Its pairs strongly irritate the mucous membranes of the eyes and upper respiratory tract. Very toxic, MPC of its content in the air is 0.7 mg / m 3 . Propenal - an intermediate product of the synthesis of certain polymers, is necessary in the production of individual drugs.
Benzaldehyde (benzoic aldehyde) 6 5 is a colorless, yellowing liquid with bitter almond flavor . It is quite rapidly oxidized by air to benzoic acid. Contained in plant essential oils (neroli, patchouli), and in the form of glucoside in the kernels of bitter almond, cherry, apricot and peach. As a fragrant, it is used in perfumery, as a component of food essences, as a raw material for the synthesis of other fragrant substances (cinnamaldehyde, jasminaldehyde).
Silver mirror reaction
Oxidation of aldehydes with silver oxide is the most significant qualitative reaction to the corresponding form of the functional group. This reaction got its name due to the thin silver coating on the walls of the test tube formed during this reaction.
Its essence lies in the interaction of the aldehyde R- with an ammonia solution of silver oxide (I), which is a soluble complex compound [Ag (NH 3 ) 2 ] OH and is called the Tollens reagent. The reaction is carried out at temperatures close to the boiling point of water (80–100 ° ). In this case, the aldehydes are oxidized to the corresponding carboxylic acids, and the oxidizing agent is reduced to metallic silver, which precipitates.
Reagent Preparation
To qualitatively determine the —COH group in aldehydes, a silver complex compound is first prepared. To do this, a little solution of ammonia (ammonium hydroxide) in water is poured into the test tube, followed by a small amount of silver nitrate. In this case, the precipitate of silver oxide immediately disappears:
2AgNO 3 + 2NH 3 + 2 -> Ag 2 O ↓ + 2NH 4 NO 3
Ag 2 O + 4NΗ 3 + Η 2 -> 2 [Ag (NΗ 3 ) 2 ] Η
Tollens reagent prepared with the addition of alkali gives more reliable results. For this, 1 g of AgNO 3 is dissolved in 10 g of distilled water and an equal volume of concentrated sodium hydroxide is added. The result is a precipitate of Ag 2 O, which disappears with the addition of a concentrated solution of ammonium hydroxide. Only a freshly prepared reagent should be used for the reaction.
Reaction mechanism
The reaction of a silver mirror corresponds to the equation:
2 [Ag (NΗ 3 ) 2 ] OΗ + Η -> 2Ag ↓ + ΗCOONΗ 4 + 3NΗ 3 + 2
It is worth noting that such an interaction has not been studied enough for aldehydes. The mechanism of this reaction is unknown, but a radical or ionic variant of oxidation is assumed. According to the diammine silver hydroxide, the addition is most likely to occur with the formation of a silver salt of a diol, from which silver is then cleaved to form a carboxylic acid.
For a successful experiment, the cleanliness of the dishes used is extremely important. This is due to the fact that the colloidal particles of silver formed during the experiment should adhere to the surface of the glass, creating a mirror surface. In the presence of the slightest contamination, it will precipitate in the form of a gray flocculent precipitate.
Alkaline solutions should be used to clean the container. So, for these purposes, you can take a solution of NaOH, which must be washed off with a large volume of distilled water. There should be no grease or mechanical particles on the glass surface.
Oxidation of copper hydroxide
The oxidation reaction of aldehydes with copper (II) hydroxide is also quite effective and effective in determining the type of functional group. It flows at a temperature corresponding to the boiling of the reaction mixture. In this case, aldehydes reduce bivalent copper in the composition of the Feling reagent (freshly prepared ammonia solution of Cu (OH) 2 ) to monovalent. They themselves are oxidized due to the introduction of an oxygen atom through the C- bond (the oxidation state of C changes from +1 to +3).
Visually, the progress of the reaction can be traced by the color change of the mixture of solutions. The bluish precipitate of copper hydroxide gradually turns yellow, corresponding to monovalent copper hydroxide and the further appearance of a bright red precipitate of Cu 2 O.
The reaction equation corresponds to this process:
R- + Cu 2+ + NaOH + 2 -> R-COONa + Cu 2 O + 4 +
Action reagent jones
It is worth noting that such a reagent acts best on aldehydes. In this case, the oxidation does not require heating and is carried out at a temperature of 0-20 ° C for a rather short period of time, and the yield of products is more than 80%. The main drawback of the Jones reagent is the lack of high selectivity for other functional groups, and besides, the acidic environment sometimes leads to isomerization or destruction.
Jones Reagent is a solution of chromium oxide (VI) in dilute sulfuric acid and acetone. It can also be obtained from sodium dichromate. During the oxidation of aldehydes, carboxylic acids are formed under the influence of this reagent.
Industrial oxygen oxidation
Oxidation of acetaldehyde in industry is carried out by exposure to oxygen in the presence of catalysts - cobalt or manganese ions. Peracetic acid is formed first:
CH 3 —COOH + O 2 -> CH 3 —COOH
It, in turn, interacts with the second molecule of acetic aldehyde and through a peroxide compound gives two molecules of acetic acid:
CH 3 —COOON + CH 3 —CON -> 2CH 3 —COOH
Oxidation is carried out at a temperature of 60-70 ° C and a pressure of 2 · 10 5 PA.
Interaction with iodine solution
For the oxidation of aldehyde groups, an iodine solution in the presence of alkali is sometimes used. This reagent is of particular importance in the process of carbohydrate oxidation, since it acts very selectively. So under his influence, D-glucose is converted into D-gluconic acid.
Iodine in the presence of alkalis forms a hypoiodide (a very strong oxidizing agent): I 2 + 2NaOΗ -> NaIO + NaI + H 2 O.
Under the action of hypoiodide, formaldehyde is converted to methanoic acid: ΗCOΗ + NaIO + NaOΗ -> ΗCOONa + NaI + H 2 O.
Oxidation of aldehydes with iodine is used in analytical chemistry to determine their quantitative content in solutions.
Selenium Oxidation
Unlike previous reagents, under the action of selenium dioxide, aldehydes are converted into dicarbonyl compounds, and glyoxal is formed from formaldehyde. If methylene or methyl groups are located near carbonyl, then they can turn into carbonyl. Dioxane, ethanol or xylene are usually used as a solvent for SeO2.
According to one of the methods, the reaction is carried out in a three-necked flask connected to a stirrer, thermometer and reflux condenser. To the starting material, taken in an amount of 0.25 mol, a solution of 0.25 mol of selenium dioxide in 180 ml of dioxane and 12 ml of H 2 O are added dropwise. The temperature should not exceed 20 ° C (if necessary, cool the flask). After that, with constant stirring, the solution is boiled for 6 hours. Next, the hot solution is filtered to separate selenium and the precipitate is washed with dioxane. After vacuum distillation of the solvent, the residue was fractionated. The main fraction is taken over a wide temperature range (20-30 ° C) and re-rectified.
Aldehyde autooxidation
Under the influence of atmospheric oxygen at room temperature, the oxidation of aldehydes occurs very slowly. The main products of these reactions are the corresponding carboxylic acids. The autooxidation mechanism is related to the industrial oxidation of ethanal to acetic acid. One of the intermediates is peracid, which interacts with another aldehyde molecule.
Due to the fact that this type of reaction is accelerated by the action of light, peroxides, and traces of heavy metals, we can conclude about its radical mechanism. Formaldehyde in aqueous solutions is much worse than its counterparts oxidized by air, due to the fact that it exists in the form of hydrated methylene glycol.
Oxidation of aldehydes with potassium permanganate
Most successfully, this reaction occurs in an acidic environment. Visually assess its passage can be the loss of intensity and complete discoloration of the pink color of the potassium permanganate solution. The reaction takes place at room temperature and normal pressure, so it does not require special conditions. It is enough to pour 2 ml of formaldehyde and 1 ml of potassium permanganate acidified with sulfuric acid into a test tube . Shake the test tube carefully to mix the reagents:
5CH 3 -COH + 2KMnO 4 + 3H 2 SO 4 = 5CH 3 -COOH + 2MnSO 4 + K 2 SO 4 + 3H 2 O
If the same reaction is carried out at elevated temperatures, then the methanal is easily oxidized to carbon dioxide:
5CH 3 -COH + 4KMnO 4 + 6H 2 SO 4 = 5CO 2 + 4MnSO 4 + 2K 2 SO 4 + 11H 2 O