A large number of organic substances that make up a living cell are characterized by large molecular sizes and are biopolymers. These include proteins that make up from 50 to 80% of the dry weight of the whole cell. Protein monomers are amino acids that bind to each other via peptide bonds. Protein macromolecules have several levels of organization and perform a number of important functions in the cell: building, protective, catalytic, motor, etc. In our article we will consider the structural features of peptides, as well as give examples of globular and fibrillar proteins that make up the human body.
Forms of organization of polypeptide macromolecules
Amino acid residues are sequentially joined together by strong covalent bonds, called peptide bonds. They are strong enough and hold in a stable state the primary structure of the protein, which has the appearance of a chain. The secondary form occurs when the polypeptide chain is twisted into an alpha helix. It is stabilized by additional hydrogen bonds. The tertiary, or native, configuration is of fundamental importance, since most globular proteins in a living cell have just such a structure. The spiral is packed in the form of a ball or globule. Its stability is caused not only by the appearance of new hydrogen bonds, but also by the formation of disulfide bridges. They arise due to the interaction of sulfur atoms that make up the cysteine amino acid. Not the least role in the formation of the tertiary structure is played by hydrophilic and hydrophobic interactions between groups of atoms within the peptide structure. If a globular protein combines with the same molecules through a non-protein component, for example, a metal ion, a quaternary configuration occurs - the highest form of organization of the polypeptide.
Fibrillar proteins
The contractile, motor and construction functions in the cell are performed by proteins whose macromolecules look like thin threads - fibrils. Polypeptides that are part of the fibers of the skin, hair, nails, belong to the fibrillar species. The most famous of them are collagen, keratin and elastin. They do not dissolve in water, but can swell in it, forming a sticky and viscous mass. Peptides of linear structure are also part of the spindle filaments, forming the mitotic apparatus of the cell. They attach to the chromosomes, contract and stretch them to the poles of the cell. This process is observed in the anaphase of mitosis - division of somatic cells of the body, as well as in the reduction and equational stages of reproduction of germ cells - meiosis. Unlike globular protein, fibrils are able to quickly stretch and contract. Cilia of ciliates, flagella, euglena of green or unicellular algae - chlamydomonas are built from fibrils and perform the functions of movement in simple organisms. The contraction of muscle proteins - actin and myosin, which are part of muscle tissue, causes a variety of skeletal muscle movements and the maintenance of the muscular skeleton of the human body.
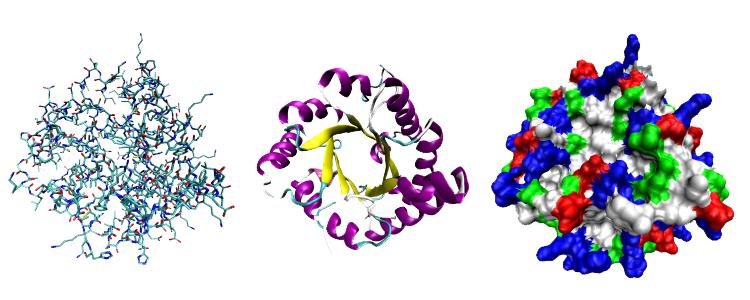
The structure of globular proteins
Peptides - carriers of molecules of various substances, protective proteins - immunoglobulins, hormones - this is an incomplete list of proteins whose tertiary structure looks like a ball - globules. In the blood there are certain proteins that have certain areas on their surface - active centers. With their help, they recognize and attach to themselves the molecules of biologically active substances produced by mixed and internal secretion glands. With the help of globular proteins, the hormones of the thyroid and gonads, adrenal glands, thymus, and pituitary gland are delivered to certain cells of the human body, equipped with special receptors for their recognition.
Membrane Polypeptides
The liquid-mosaic model of the structure of cell membranes is best suited to perform important functions: barrier, receptor, and transport. The proteins included in it transport ions and particles of certain substances, for example, glucose, amino acids, etc. The properties of globular carrier proteins can be studied using the sodium-potassium pump as an example. It carries out the transition of ions from the cell into the intercellular space and vice versa. Sodium ions are constantly moving in the middle of the cell cytoplasm, and potassium cations are moving out of the cell. Violation of the desired concentration of these ions leads to cell death. To prevent this threat, a special protein is built into the cell membrane. The structure of globular proteins is such that they transfer Na + and K + cations against a concentration gradient using the energy of adenosine triphosphoric acid.
The structure and functions of insulin
Soluble spherical proteins in tertiary form serve as metabolic regulators in the human body. The insulin produced by the beta cells of the islets of Langerhans controls blood glucose levels. It consists of two polypeptide chains (α- and β-forms) connected by several disulfide bridges. These are covalent bonds arising between the molecules of a sulfur-containing amino acid - cysteine. The pancreatic hormone mainly consists of an ordered sequence of amino acid units organized in the form of an alpha helix. An insignificant part of it has the form of a β-structure and amino acid residues without strict spatial orientation.
Hemoglobin
A classic example of globular peptides is the blood protein, which determines the red color of the blood - hemoglobin. The protein contains four polypeptide sites in the form of an alpha and beta helix, which are connected by a non-protein component - heme. It is represented by an iron ion that binds polypeptide chains into a single confirmation related to the Quaternary form. Oxygen particles (in this form it is called oxyhemoglobin) are attached to the protein molecule and are then transported to the cells. This ensures the normal course of the processes of dissimilation, since in order to obtain energy, the cell oxidizes the organic substances that enter it.
The role of blood protein in gas transport
In addition to oxygen, hemoglobin is also able to attach carbon dioxide. Carbon dioxide is formed as a by-product of catabolic cellular reactions and must be removed from the cells. If carbon monoxide, carbon monoxide, is present in the inhaled air, it is able to form a strong connection with hemoglobin. In this case, a toxic substance without color and smell during breathing quickly penetrates the cells of the body, causing poisoning. Brain structures are particularly sensitive to high concentrations of carbon monoxide. There is paralysis of the respiratory center located in the medulla oblongata, which entails death from suffocation.
In our article, we examined the structure, structure, and properties of peptides, as well as examples of globular proteins that perform a number of important functions in the human body.