Discoveries in the field of atomic structure became an important step in the development of physics. Rutherford's model was of great importance. An atom as a system and particles, its components, has been studied more accurately and in detail. This led to the successful establishment of a science such as nuclear physics.
Antique notions of the structure of matter
The assumption that the surrounding bodies are composed of the smallest particles were made back in ancient times. The thinkers of that time represented the atom as the smallest and indivisible particle of any substance. They argued that there is nothing smaller in the universe than an atom. Such views were held by the great ancient Greek scientists and philosophers - Democritus, Lucretius, Epicurus. The hypotheses of these thinkers today are united under the name of "ancient atomism."
Medieval performance
The times of antiquity passed, and in the Middle Ages there were also scientists who expressed various assumptions about the structure of substances. However, the prevalence of religious philosophical views and the power of the church in that period of history completely suppressed any attempts and aspirations of the human mind to materialistic scientific conclusions and discoveries. As you know, the medieval Inquisition behaved very unfriendly with representatives of the scientific world of that time. It remains to say that the then bright minds had an idea from the antiquity of the indivisibility of the atom.
Research 18-19 centuries
The 18th century was marked by major discoveries in the field of the elementary structure of matter. Largely thanks to the efforts of scientists such as Antoine Lavoisier, Mikhail Lomonosov and John Dalton. Independently of each other, they were able to prove that atoms really exist. But the question of their internal structure remained open. The end of the 18th century was marked by such a significant event in the scientific world as the discovery by D. I. Mendeleev of the periodic system of chemical elements. This was a truly powerful breakthrough of that time and opened the curtain on the understanding that all atoms have a single nature, that they are related to each other. Later, in the 19th century, another important step towards unraveling the structure of the atom was the proof that an electron is present in any of them. The work of scholars of this period prepared fertile ground for the discoveries of the 20th century.
Thomson's experiments
The English physicist John Thomson in 1897 proved that the composition of atoms includes electrons with a negative charge. At this stage, the false notion that the atom is the fissility limit of any substance was finally destroyed. How did Thomson manage to prove the existence of electrons? The scientist in his experiments placed electrodes in very rarefied gases and passed an electric current. As a result, cathode rays arose. Thomson carefully studied their features and found that they are a stream of charged particles that move at great speed. The scientist was able to calculate the mass of these particles and their charge. He also found that they cannot be converted into neutral particles, because the electric charge is the basis of their nature. So the electrons were discovered. Thomson is also the creator of the world's first atomic structure model. According to her, an atom is a bunch of positively charged matter in which negatively charged electrons are evenly distributed. Such a structure explains the general neutrality of atoms, since opposite charges balance each other. The experiments of John Thomson became invaluable for further study of the structure of the atom. However, many questions remained unanswered.
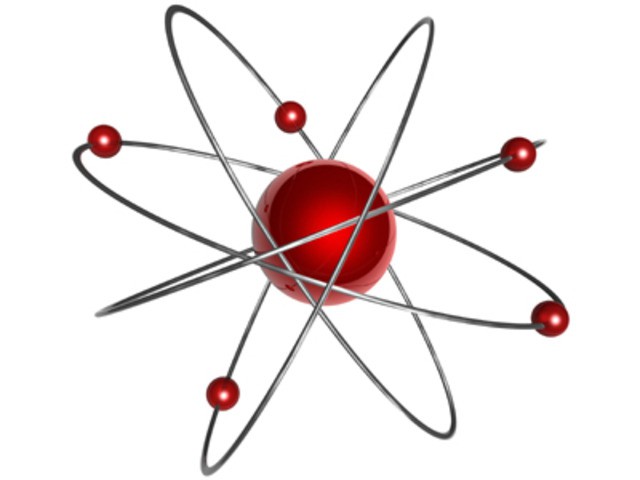
Rutherford Research
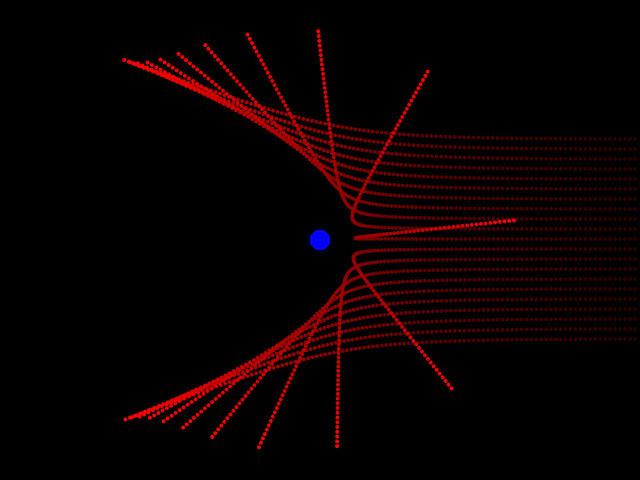
Thomson discovered the existence of electrons, but he was unable to find positively charged particles in the atom. Ernest Rutherford corrected this misunderstanding in 1911. During experiments, studying the activity of alpha particles in gases, he discovered that positively charged particles are present in the atom. Rutherford saw that as the rays passed through the gas or through a thin metal plate, a small number of particles abruptly deviate from the trajectory. They were literally thrown back. The scientist guessed that this behavior is explained by a collision with positively charged particles. Such experiments allowed the physicist to create a model of the structure of the atom of Rutherford.
Planetary model
Now the scientist's ideas were somewhat different from the assumptions made by John Thomson. Different were their atomic models. Rutherford's experience allowed him to create a completely new theory in this area. The discoveries of the scientist were crucial for the further development of physics. Rutherford's model describes an atom as a core located in the center, and electrons moving around it. The nucleus has a positive charge, and the electrons have a negative charge. The Rutherford model of the atom assumed the rotation of electrons around the nucleus along certain trajectories - orbits. The discovery of the scientist helped to explain the reason for the deviation of alpha particles and became the impetus for the development of the nuclear theory of the atom. In the Rutherford atomic model, an analogy is traced with the movement of the planets of the solar system around the sun. This is a very accurate and vivid comparison. Therefore, the Rutherford model, the atom in which moves around the nucleus in orbit, was called planetary.
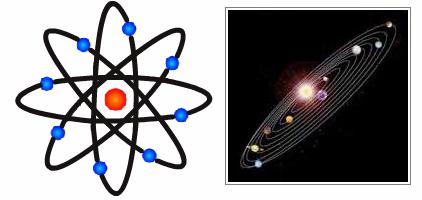
Niels Bohr Works
Two years later, the Danish physicist Niels Bohr tried to combine ideas about the structure of the atom with the quantum properties of the light flux. Rutherford's nuclear model of the atom was laid by the scientist at the basis of his new theory. According to Bohr, atoms rotate around the nucleus in circular orbits. Such a trajectory of motion leads to acceleration of electrons. In addition, the Coulomb interaction of these particles with the center of the atom is accompanied by the creation and expenditure of energy to maintain the spatial electromagnetic field arising from the motion of electrons. Under such conditions, negatively charged particles should someday fall onto the nucleus. But this does not happen, which indicates a greater stability of atoms as systems. Niels Bohr realized that the laws of classical thermodynamics described by Maxwell's equations do not work under intra-atomic conditions. Therefore, the scientist set himself the task of deriving new laws that would be valid in the world of elementary particles.
Postulates of Bohr
Largely due to the fact that there was a Rutherford model, the atom and its components were well studied, Niels Bohr was able to approach the creation of his postulates. The first of them says that an atom has stationary states in which it does not change its energy, while electrons move in their orbits, without changing their path. According to the second postulate, when an electron transfers from one orbit to another, energy is released or absorbed. It is equal to the difference of energies of the previous and subsequent states of the atom. Moreover, if an electron jumps to an orbit closer to the nucleus, then radiation of energy (photon) occurs , and vice versa. Despite the fact that the motion of the electrons does not resemble the orbital trajectory located strictly around the circumference, the discovery of Bohr has provided an excellent explanation for the existence of the line spectrum of the hydrogen atom. Around the same time, physicists Hertz and Frank, who lived in Germany, confirmed the teachings of Niels Bohr on the existence of stationary, stable states of an atom and the possibility of changing the values of atomic energy.

The collaboration of two scientists
By the way, Rutherford for a long time could not determine the charge of the nucleus. Scientists Marsden and Geiger tried to double-check the statements of Ernest Rutherford and, as a result of detailed and thorough experiments and calculations, came to the conclusion that the nucleus is the most important characteristic of the atom, and its entire charge is concentrated in it. It was further proved that the value of the nuclear charge is numerically equal to the ordinal number of an element in the periodic system of elements of D. I. Mendeleev. Interestingly, Niels Bohr soon met Rutherford and completely agreed with his views. Subsequently, scientists worked for a long time together in one laboratory. Rutherford's model, an atom as a system consisting of elementary charged particles, - Niels Bohr considered all this to be fair and forever set aside his electronic model. The joint scientific activity of scientists was very successful and bore fruit. Each of them delved into the study of the properties of elementary particles and made discoveries significant for science. Rutherford later discovered and proved the possibility of decomposition of the core, but this is the topic of another article.