Every day we use some objects: we take them in our hands, we perform any manipulations on them - we turn them over, consider them, finally break them. Have you ever thought about what these items consist of? "What can I think here? Of metal / wood / plastic / fabric!" - Many of us will bewildered. This is partly the correct answer. And what do these materials consist of - metal, wood, plastic, fabric and many other substances? Today we will discuss this issue.
Molecule and Atom: Definition
For a knowledgeable person, the answer to it is simple and banal: from atoms and molecules. But some people are puzzled and start to pour questions: "What is an atom and a molecule? How do they look?" etc. We will answer these questions in order. Well, first of all, what is an atom and a molecule? Let us tell you right away that these definitions are not the same thing. And even more than that, these are completely different terms. So, an atom is the smallest part of a chemical element, which is the carrier of its properties, a particle of substance of scanty mass and size. And a molecule is an electrically neutral particle, which is formed by several atoms connected by covalent bonds .
What is an atom: structure
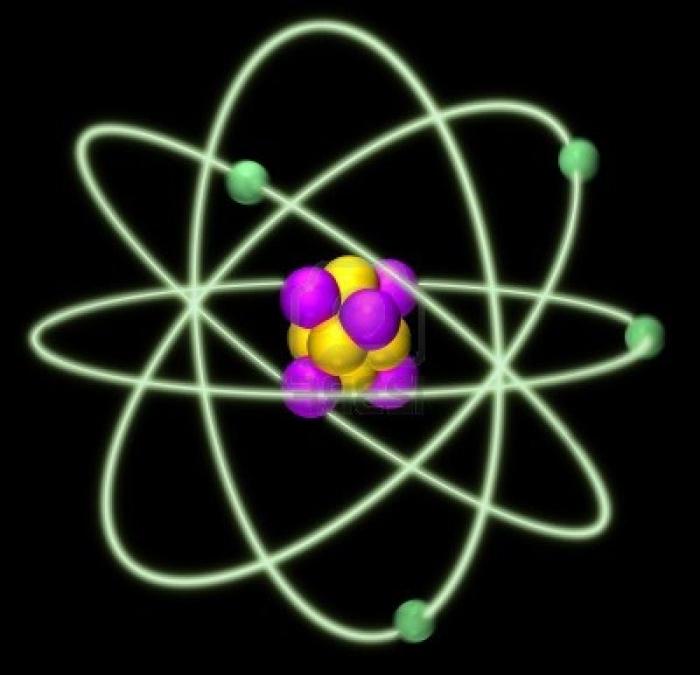
An atom consists of an electron shell and an atomic nucleus (photo). In turn, the nucleus consists of protons and neutrons, and the shell - of electrons. In an atom, protons are positively charged, electrons are negatively charged, and neutrons are not charged at all. If the number of protons corresponds to the number of electrons, then the atom is electrically neutral, i.e. if we touch a substance formed from molecules with such atoms, then we will not feel the slightest electrical impulse. And even heavy duty computers will not catch him because of the lack of the latter. But it happens that there are more protons than electrons, and vice versa. Then such atoms would more correctly be called ions. If there are more protons in it, then it is electrically positive; if electrons prevail, it is electrically negative. Each specific atom has a strict number of protons, neutrons and electrons. And it can be calculated. The template for solving the problems of finding the number of these particles looks like this:
Chem. element - R (insert element name)
Protons (p) -?
Electrons (e) -?
Neutrons (n) -?
Decision:
p = serial number chem. element R in the periodic system named after D.I. Mendeleev
e = p
n = A r (R) - No. R
What is a molecule: structure
A molecule is the smallest particle of a chemical substance, that is, it is already directly included in its composition. A molecule of a certain substance consists of several identical or different atoms. The structural features of the molecules depend on the physical properties of the substance in which they are present. Molecules are made up of electrons and atoms. The location of the latter can be found using the structural formula. The structure of the molecule allows you to determine the course of a chemical reaction. Usually they are neutral (do not have an electric charge), and they do not have unpaired electrons (all valencies are saturated). However, they can be charged, then their correct name is ions. Also, molecules can have unpaired electrons and unsaturated valencies - in this case they are called radicals.
Conclusion
Now you know what an atom is and
what a molecule is. Without exception, all substances are composed of molecules, and the latter, in turn, are built of atoms. The physical properties of a substance determine the location and bonding of atoms and molecules in it.