The discovery of an electron has once again raised the question for scientists around the world: what is the internal structure of an atom? Naturally, it is impossible to discern even the most powerful microscope how everything is arranged there. Therefore, various scientists proposed their versions of the internal structure of the atom.
So, J. Thompson proposed a model according to which the atom entirely consisted of a positively charged substance, inside which negatively charged electrons constantly moved. In parallel with Thompson, F. Lenard at the beginning of the twentieth century suggested that there is a void inside an atom along which neutral particles consisting of the same number of electrons and some positively charged elements move. In the works of Lenard, these particles were called dynamides.
However, the most comprehensive was the so-called planetary model of the Rutherford atom. A series of experiments on uranium made this scientist truly famous, as a result of which such a phenomenon as radioactivity was formulated and theoretically explained.
Just thinking that the planetary model of the atom is the true expression of the structure of this element, in his first major scientific studies, Rutherford came to the conclusion that the energy hidden inside the atom is several tens of thousands of times greater than molecular energy. From this conclusion, he went on to explain some cosmic phenomena, stating, in particular, that solar energy is nothing but the result of constant reactions, including the splitting of an atom.
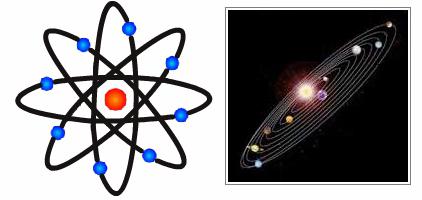
The most important step towards understanding the structure of the atom was the famous experiments on the movement of alpha particles through gold foil: the vast majority of these particles passed through it without any changes, but individual elements sharply deviated from their route. Rutherford suggested that in this case, these particles pass next to like-charged elements, the sizes of which are much smaller than the size of an atom. So the famous planetary model of the structure of the atom was born . This was a great achievement of the scientist.
The planetary model of the atom was proposed at the very beginning of the twentieth century by J. Stoney, but he had it exclusively theoretical, while Rutherford came to it through experiments, the results of which were published in 1911 in the Philosophical Journal.
Continuing his experiments, Rutherford came to the conclusion that the number of alpha particles fully corresponds to the ordinal number of the element in the recently published periodic table of Mendeleev. In parallel, the Danish scientist Niels Bohr, creating his theory of metals, made an important discovery concerning the orbits of the motion of electrons, which became one of the most important evidence that it is the planetary model of the atom that is closest to the real structure of this elementary particle. Opinions of scientists coincided.
Thus, the planetary model of the atom is a theoretical justification for the structure of this elementary particle, according to which the center of the atom has a nucleus with protons whose charge is positive and electrically neutral neutrons, and around the nucleus, at a considerable distance from it, they move negatively in orbits charged electrons.