All modern car exhaust systems include a catalytic converter. This device is designed to reduce the level of emission of harmful substances with exhaust gases into the atmosphere. The catalytic converter is used both on diesel power units, and on gasoline. Install it either immediately after the exhaust manifold, or immediately before the muffler. The exhaust gas neutralizer consists of a carrier unit, thermal insulation, and housing.
Device
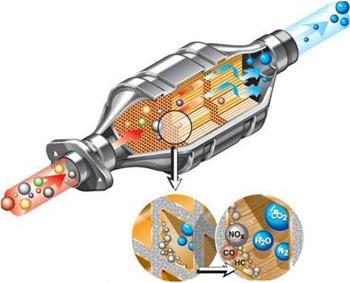
The main element is the block carrier. It is made of refractory ceramics. The design of such a unit consists of a large number of longitudinal cells, significantly increasing the area of contact with the exhaust gases. Their surface is coated with special catalyst substances (palladium, platinum and rhodium). Thanks to these elements, chemical reactions are accelerated.
Palladium and platinum are oxidizing catalysts. They provide the oxidation of hydrocarbons and, accordingly, contribute to their conversion to carbon monoxide, carbon dioxide and water vapor. And rhodium is a reducing catalyst. It is used to reduce nitrogen oxides to harmless nitrogen. It turns out that three types of catalysts reduce the content of three different harmful substances in the exhaust gases. Therefore, such a device was called a three-component catalytic converter.
The carrier block is housed in a metal case. Between them is a heat-insulating layer. In the catalyst housing is an oxygen sensor.
The effective operation of the device in question is achieved at a temperature of 300 ° C; in this case, about 90 percent of harmful substances are delayed (for this, the catalytic converter is installed immediately after the exhaust manifold).
Features
Catalysts quite effectively reduce the toxicity of exhaust gases and at the same time practically do not affect engine power and fuel consumption. With this device, the back pressure will increase slightly, as a result of which the power unit of the car loses 2-3 liters. from. Theoretically, the exhaust catalyst can last forever, because precious metals are not consumed during chemical reactions. However, as practice shows, the service life of these devices has its limit.
For example, one of the most common reasons for the failure of the neutralizers is the fragile ceramic of the cells, which can collapse due to a sharp shock (if the car hits the speed, hits a pothole or even strikes the catalyst body for something), which leads to failure the mentioned device. Now converters began to appear, in which instead of ceramics - a metal monolith. They are more resistant to destruction. Another reason for catalytic converter failure is fuel. Leaded gasoline is rich in tetraethyl lead, which “greases” the surface of the cells. As a result, all reactions cease. The next enemy of the catalyst is the wrong fuel composition. So, a mixture containing an increased amount of hydrocarbons simply ruins the device, and too lean causes a sharp overheat, which can lead to the destruction of the monolith. Sharp changes in temperature are no less dangerous, for example, when a car enters a puddle. It can also damage the ceramic.
In general, the catalytic converter, like any other mechanism, is affected by operating conditions.