All life on the planet consists of many cells that maintain the orderliness of their organization due to the genetic information contained in the core. It is stored, realized and transmitted by complex macromolecular compounds - nucleic acids, consisting of monomeric units - nucleotides. The role of nucleic acids cannot be overestimated. The stability of their structure determines the normal functioning of the body, and any deviations in the structure will inevitably lead to a change in cellular organization, the activity of physiological processes and the viability of cells in general.
The concept of nucleotide and its properties
Each DNA or RNA molecule is assembled from smaller monomeric compounds - nucleotides. In other words, a nucleotide is a building material for nucleic acids, coenzymes, and many other biological compounds that are essential for the cell during its life.
The main properties of these essential substances include:
• storage of information on protein structure and heritable traits;
• control over growth and reproduction;
• participation in metabolism and many other physiological processes taking place in the cell.
Nucleotide composition
Speaking of nucleotides, one cannot help but dwell on such an important issue as their structure and composition.
Each nucleotide consists of:
• sugar residue;
• nitrogen base;
• phosphate group or phosphoric acid residue.
We can say that the nucleotide is a complex organic compound. Depending on the species composition of nitrogenous bases and the type of pentose in the nucleotide structure, nucleic acids are divided into:
• deoxyribonucleic acid, or DNA;
• ribonucleic acid, or RNA.
The composition of nucleic acids
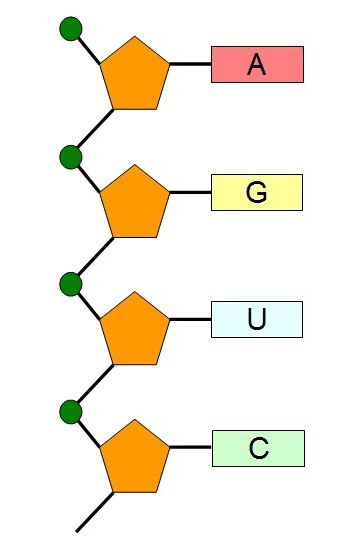
In nucleic acids, sugar is represented by pentose. This is five-carbon sugar, in DNA it is called deoxyribose, in RNA - ribose. Each pentose molecule has five carbon atoms, four of them together with an oxygen atom form a five-membered ring, and the fifth is included in the HO-CH2 group.
The position of each carbon atom in the pentose molecule is indicated by an Arabic numeral with a prime (1C´, 2C´, 3C´, 4C´, 5C´). Since all processes of reading hereditary information from a nucleic acid molecule are strictly directed, the numbering of carbon atoms and their location in the ring serve as a kind of indicator of the correct direction.
At the hydroxyl group, a phosphoric acid residue is attached to the third and fifth carbon atoms (3C´ and 5C´). It determines the chemical belonging of DNA and RNA to the group of acids.
A nitrogen base is attached to the first carbon atom (1C´) in the sugar molecule.
Species composition of nitrogenous bases
Nitrogen base DNA nucleotides are represented by four species:
• adenine (A);
• guanine (G);
• cytosine (C);
• thymine (T).
The first two belong to the class of purines, the last two - pyrimidines. By molecular weight, purines are always heavier than pyrimidines.
Nitrogen base RNA nucleotides are represented by:
• adenine (A);
• guanine (G);
• cytosine (C);
• uracil (U).
Uracil, like thymine, is a pyrimidine base.
In the scientific literature, one can often find another designation of nitrogenous bases - in Latin letters (A, T, C, G, U).
Let us dwell on the chemical structure of purines and pyrimidines.
Pyrimidines, namely cytosine, thymine and uracil, are composed of two nitrogen atoms and four carbon atoms forming a six-membered ring. Each atom has its number from 1 to 6.
Purines (adenine and guanine) are composed of pyrimidine and imidazole or two heterocycles. The purine base molecule is represented by four nitrogen atoms and five carbon atoms. Each atom is numbered from 1 to 9.
As a result of the combination of the nitrogenous base and the pentose residue, a nucleoside is formed. A nucleotide is a compound of a nucleoside and a phosphate group.
The formation of phosphodiester bonds
It is important to understand the question of how nucleotides are combined into a polypeptide chain and form a nucleic acid molecule. This happens due to the so-called phosphodiester bonds.
The interaction of two nucleotides gives a dinucleotide. The formation of a new compound occurs by condensation, when a phosphodiester bond occurs between the phosphate residue of one monomer and the pentose hydroxy group of the other.
Synthesis of polynucleotide - repeated repetition of this reaction (several million times). The polynucleotide chain is built through the formation of phosphodiester bonds between the third and fifth carbons of sugars (3C´ and 5C´).
The assembly of the polynucleotide is a complex process involving the DNA polymerase enzyme, which provides chain growth from only one end (3´) with a free hydroxy group.
DNA molecule structure
A DNA molecule, like a protein, can have a primary, secondary, and tertiary structure.
The nucleotide sequence in a DNA strand determines its primary structure. The secondary structure is formed due to hydrogen bonds, which are based on the principle of complementarity. In other words, a certain pattern applies in the synthesis of the DNA double helix : adenine of one chain corresponds to thymine of the other, guanine to cytosine, and vice versa. Pairs of adenine and thymine or guanine and cytosine are formed due to two in the first and three in the latter case, hydrogen bonds. This combination of nucleotides provides a strong bond of the chains and an equal distance between them.
Knowing the nucleotide sequence of one DNA strand, by the principle of complementarity or complement, the second can be completed.
The tertiary structure of DNA is formed due to complex three-dimensional bonds, which makes its molecule more compact and able to accommodate in a small cell volume. For example, the DNA length of Escherichia coli is more than 1 mm, while the cell length is less than 5 microns.
The number of nucleotides in DNA, namely their quantitative ratio, obeys the Chergaff rule (the number of purine bases is always equal to the number of pyrimidine bases). The distance between the nucleotides is a constant value equal to 0.34 nm, as well as their molecular weight.
RNA molecule structure
RNA is represented by a single polynucleotide chain formed through covalent bonds between pentose (in this case, ribose) and a phosphate residue. In length, it is much shorter than DNA. According to the species composition of nitrogenous bases, there are also differences in the nucleotide. In RNA, uracil is used instead of the pyrimidine base of thymine. Depending on the functions performed in the body, RNA can be of three types.
• Ribosomal (rRNA) - usually contains from 3,000 to 5,000 nucleotides. As a necessary structural component, it takes part in the formation of the active center of ribosomes, the site of one of the most important processes in the cell - protein biosynthesis.
• Transport (tRNA) - consists of an average of 75 - 95 nucleotides, transfers the desired amino acid to the site of polypeptide synthesis in the ribosome. Each type of tRNA (at least 40) has its own sequence, unique to it, of monomers or nucleotides.
• Information (mRNA) - the nucleotide composition is very diverse. It transfers genetic information from DNA to ribosomes, acts as a matrix for the synthesis of a protein molecule.
The role of nucleotides in the body
Nucleotides in the cell perform a number of important functions:
• are used as building blocks for nucleic acids (nucleotides of purine and pyrimidine series);
• participate in many metabolic processes in the cell;
• are part of ATP - the main source of energy in cells;
• act as carriers of reducing equivalents in cells (NAD +, NADP +, FAD, FMN);
• perform the function of bioregulators;
• can be considered as second messengers of extracellular regular synthesis (for example, cAMP or cGMP).
A nucleotide is a monomeric unit that forms more complex compounds - nucleic acids, without which the transfer of genetic information, its storage and reproduction is impossible. Free nucleotides are the main components involved in signaling and energy processes that support the normal functioning of cells and the body as a whole.