Among the information on the problem of proper nutrition, one can distinguish a segment that reveals the effect on the human body of substances such as nitrates. Their effect on our health is ambiguous. How not to recall the famous expression of Paracelsus: “Everything is poison and everything is medicine; one dose or another, only the dose makes it. ” Indeed, for proper metabolism, the presence of NO 3 - NO 4 + anions in the cell cytoplasm is necessary. At the same time, an excess of compounds containing these ions in food is dangerous to human health. The role of nitric acid salts, their preparation, and the use of nitrates will be considered in this article.
The position of nitrates in the classification of inorganic substances
Many people know that nitric acid salts, which are highly soluble in water, are nitrates. What is saltpeter is not known to everyone. It turns out that earlier in chemistry they called special substances - salts of nitrate acid. Until now, we can hear this term in relation to the chemistry of mineral fertilizers. In nitrate nitrate ion is associated with cations of potassium, calcium, sodium and ammonium. They are medium salts. It turned out that sodium, potassium nitrates and other salts decompose upon heating, and the reaction products depend on which cation is part of the molecule. So, nitrates, the formula of which is KaNO 3 or NaNO 3 are thermally decomposed to the corresponding nitrite and free oxygen, while magnesium nitrate forms oxide, nitrogen dioxide and oxygen when heated.
Nitrate production
The main method is the processing of natural minerals containing nitric acid salts. So, Chilean nitrate, which is a mineral nitronatrite, is one of the main sources of their production. At plants of the fertilizer industry, the rock containing sodium nitrate is finely ground and packaged is delivered to the farm enterprises as nitrogen fertilizer. It is used primarily for increasing the vegetative mass of vegetables, grains and fruit crops, as well as to increase their productivity. In laboratory conditions, nitric acid salts are obtained by an exchange reaction between alkalis and nitric acid or by the interaction of metals with concentrated HNO 3. The use of nitrogen fertilizers, such as potassium nitrate, ammonium nitrate, is a necessary condition for obtaining stable high yields of main crops.
Agrotechnical conditions for the application of nitrogen fertilizers (nitrates)
These include, first of all, compliance with the standards for applying drugs to the soil. For example, for ammonium nitrate containing up to 35% nitrogen, the permissible rate is not more than 25 g per square meter. Calcium nitrate, which contains up to 15.5% of nitrogen, is applied at the rate of up to 50 g per 1 m. Violation of agrotechnical norms of nitrogen fertilizing of plants leads not only to soil contamination and a decrease in its restoration function, but also to a dangerous concentration of compounds such as nitrates, in vegetables and fruits. We will dwell on this issue further.
How nitrates get into plants
Consider exactly how nitrates enter the soil, what is pre-sowing application, starting fertilizer and top dressing. In agriculture, the main fertilization is carried out in the spring and at the same time pay attention to the composition of the soil and climate features. The goal is to provide nitrogen nutrition during the growing season of the plant.
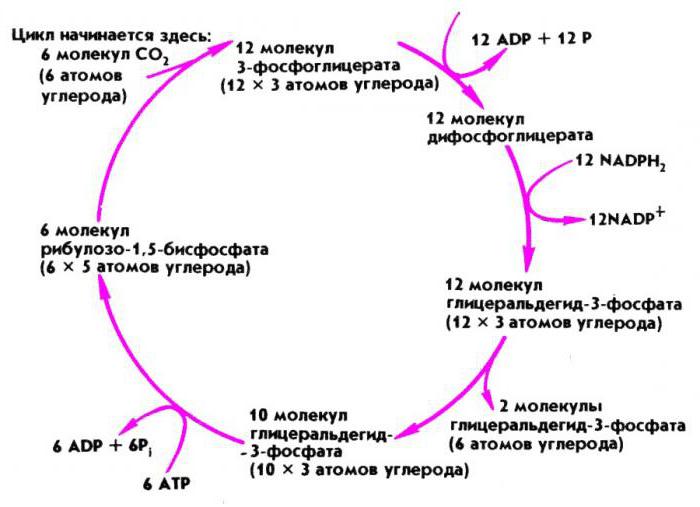
When using the starting method, nitrate is brought directly into rows or wells, using nitrates in minimal doses so as not to “burn” the fragile roots of the planted seedlings. Feeding is carried out during the most intensive absorption of nutrients by plants. It is carried out in the form of vegetative crops or as root top dressing with nitrate solutions. In the soil substrate, ammonium ions or nitrate ions by diffusion are easily absorbed by the root hairs and then enter the xylem. Conductive elements of plants consist of it. In gymnosperms, these are tracheids, and in flowering ones, vessels (trachea). Nitrogen atoms, which are part of nitrates, are included in the Calvin cycle, which is carried out in the dark phase by photosynthesis, and are subsequently included in the synthesized amino acids and further in plant proteins.
The danger of excess nitrate acid salts
If the concentration of the above compounds exceeds the permissible norm, this can determine the device for measuring nitrates in products. It is commercially available and used in many families responsible for their health. Medical studies have shown that an excess of HNO 3 salts in the gastrointestinal tract turns into nitrites. They cause irritation of the mucous membranes of the stomach and duodenum, symptomatically manifested by the phenomena of dyspepsia and food poisoning. It was also found that excess salts of HNO 2 are poorly excreted by the kidneys, so they accumulate in the renal parenchyma. It is known that the kidneys themselves play a leading role in the metabolism and excretion of harmful metabolic products. Reduced renal filtration function is a very dangerous complication arising from poisoning with nitric and nitrous acid salts . As a result of eating foods high in nitrates, the liver, which is another detoxifying organ, also suffers. Its damage with nitrates is fraught with severe damage up to the degeneration of hepatocytes into adipose tissue.

Oxygen starvation of tissues as a consequence of their nitrate poisoning
Nutrients (fats, carbohydrates) can be stored as a reserve source of energy and are consumed gradually by the body. That is why a person is able to do without food for 5-7 days. But oxygen, unlike carbohydrates and fats, cannot be stored for a long time in cells or tissues, so hypoxia within 5-15 minutes leads to the death of brain cells, and then the entire human body as a whole. Seemingly harmless at first glance, nitrates in fruits, vegetables, and infant formula can cause the destruction of the respiratory chains in the mitochondria, disrupt the formation of the connection of the erythrocyte pigment - hemoglobin - with oxygen molecules.
And impede its transfer to organs and tissues. Therefore, an excessive concentration in the cytosol of the cell of such compounds as nitrates (formula - Me (NO 3 ) N 1) can lead to serious impairment of functions on the part of physiological systems such as digestive, excretory, and also nervous.
What foods may be contaminated with nitrate salts
In most people, an excess of these substances is associated with vegetables or fruits, such as watermelon, potatoes, radishes or strawberries. But it turns out that pollution of the soil and especially water with nitrogen fertilizers that got there due to the violation of the norms of their application directly entails the "sedimentation of nitrates" in the organisms of domestic animals. Therefore, the uncontrolled and irrational use of nitrates entails their high content in animal products: meat, milk and even honey. Spraying honey plants (buckwheat, rapeseed) with solutions of nitric acid salts as a liquid fertilizer leads to the appearance of toxins in beekeeping products.
How to determine the content of nitric acid salts in products
Let's clarify how it is practically possible to learn about the concentration of compounds such as nitrates in food substances. What is a nitrate tester, consider in more detail. To detect the presence of nitric acid salts, you need to immerse the probe in the pulp of a watermelon or other product. The device has two parts: positively and negatively charged. And the nitratomer itself determines the electrical conductivity of the solution of cell juice. The results are compared with the MPC of the product under study, and the device for measuring nitrates in the products displays the result with the salt content in mg / kg. Particularly desirable will be testing foods used in the nutrition of young children, pregnant and lactating women, as well as people suffering from chronic diseases of the gastrointestinal tract and kidneys.
Poisoning with nitrate salts and its prevention
The peak of recorded cases of intoxication associated with poisoning with nitric acid salts occurs at the end of winter - the beginning of spring. Due to the lack of sunlight and energy, the human body during this period is in dire need of vitamins contained in fresh vegetables and fruits. Therefore, in the supermarkets greenhouse cucumbers, tomatoes, spicy and flavoring condiments (celery, parsley, dill) and strawberries do not lie. Agricultural technologies for their cultivation provide for the mandatory use of substances such as nitrates. What are mineral fertilizers and how their excess affects the human body, has already been considered by us earlier. Now we will focus on first aid techniques that can alleviate the condition of the patient with symptoms of nitrate poisoning. First of all, you need to induce vomiting in order to remove a toxic product from the stomach. To do this, use a plentiful drink. Then take enterosorbents and continue to comply with the water-salt regime. For complex symptoms, such as a drop in blood pressure, loss of consciousness, cramps caused by damage to the central nervous system and kidneys, an ambulance should be called urgently.