Many substitution reactions pave the way for the production of a variety of compounds with economic applications. A huge role in chemical science and industry is given to electrophilic and nucleophilic substitution. In organic synthesis, these processes have a number of features that should be paid attention to.
A variety of chemical phenomena. Substitution Reactions
Chemical changes associated with the transformation of substances differ in a number of features. The final results, thermal effects may be different; some processes go to the end, in others chemical equilibrium sets in . A change in substances is often accompanied by an increase or decrease in the degree of oxidation. When classifying chemical phenomena according to their final result, attention is paid to the qualitative and quantitative differences of reagents from products. Based on these characteristics, 7 types of chemical transformations can be distinguished, including substitution proceeding according to the scheme: A — B + C A — C + B. A simplified record of a whole class of chemical phenomena gives an idea that among the starting materials there is a so-called “attacking” "A particle replacing an atom, an ion, a functional group in a reagent. The substitution reaction is characteristic of saturated and aromatic hydrocarbons.
Substitution reactions can occur in the form of a double exchange: A — B + C — E A — C + B — E. One of the subspecies is the displacement, for example, of copper by iron from a solution of copper sulfate: CuSO 4 + Fe = FeSO 4 + Cu. As an “attacking” particle, atoms, ions or functional groups can act
Homolytic substitution (radical, SR)
With the radical mechanism of breaking covalent bonds, the electron pair, common to different elements, is proportionally distributed between the "fragments" of the molecule. Free radicals are formed. These are unstable particles, the stabilization of which occurs as a result of subsequent transformations. For example, upon the production of ethane from methane, free radicals appear which are involved in the substitution reaction: CH 4 CH 3 • + • H; CH 3 • + • CH 3 → C2H5; H • + • H → H2. Homolytic bond breaking according to the given substitution mechanism is characteristic of alkanes, the reaction is of a chain nature. In methane, H atoms can be replaced successively with chlorine. A similar reaction occurs with bromine, but iodine is not able to directly replace hydrogen in alkanes, fluorine reacts too energetically with them.
Heterolytic bond cleavage
With the ionic mechanism of substitution reactions, the electrons are unevenly distributed between the newly formed particles. A binding pair of electrons goes completely to one of the "fragments", most often, to that partner in the bond, towards which the negative density in the polar molecule was shifted. Substitution reactions include the formation of methyl alcohol CH 3 OH. In CH3Br bromomethane, the molecular break is heterolytic, and the charged particles are stable. Methyl gets a positive charge, and bromine gets a negative charge: CH 3 Br → CH 3 + + Br - ; NaOH → Na + + OH - ; CH 3 + + OH - → CH 3 OH; Na + + Br - ↔ NaBr.
Electrophiles and nucleophiles
Particles that lack electrons and can accept them are called "electrophiles." These include carbon atoms bonded to halogens in haloalkanes. Nucleophiles have an increased electron density, they “donate” a pair of electrons to create a covalent bond. In substitution reactions, nucleophiles rich in negative charges are attacked by electrophiles lacking electrons. This phenomenon is associated with the movement of an atom or other particle - a leaving group. Another type of substitution reaction is an electrophile attack by a nucleophile. It is sometimes difficult to distinguish between two processes, to attribute substitution to one or another type, since it is difficult to accurately indicate which of the molecules is the substrate and which is the reagent. Typically, in such cases, the following factors are taken into account:
- the nature of the leaving group;
- nucleophile reactivity;
- the nature of the solvent;
- structure of the alkyl part.
Nucleophilic Substitution (SN)
In the process of interaction in an organic molecule, an increase in polarization is observed. In the equations, a partial positive or negative charge is marked with the letter of the Greek alphabet. The polarization of the bond allows one to judge the nature of its breaking and the further behavior of the “fragments” of the molecule. For example, the carbon atom in iodomethane has a partial positive charge, is an electrophilic center. It attracts that part of the water dipole where oxygen is located, which has an excess of electrons. During the interaction of the electrophile with a nucleophilic reagent, methanol is formed: CH 3 I + H 2 O → CH 3 OH + HI. Nucleophilic substitution reactions take place with the participation of a negatively charged ion or molecule with a free electron pair that is not involved in creating a chemical bond. The active participation of iodomethane in SN 2 reactions is explained by its openness to nucleophilic attack and the mobility of iodine.
Electrophilic Substitution (SE)
A nucleophilic center may be present in an organic molecule, which is characterized by an excess of electron density. It reacts with a negatively charged electrophilic reagent. Such particles include atoms having free orbitals, molecules with regions of reduced electron density. In sodium formate, carbon having a “-” charge interacts with the positive part of the water dipole — with hydrogen: CH 3 Na + H 2 O → CH 4 + NaOH. The product of this electrophilic substitution reaction is methane. In heterolytic reactions, oppositely charged centers of organic molecules interact, which makes them similar to ions in the chemistry of inorganic substances. It should not be overlooked that the conversion of organic compounds is rarely accompanied by the formation of true cations and anions.
Monomolecular and bimolecular reactions
Nucleophilic substitution is monomolecular (SN1). According to this mechanism, hydrolysis of an important product of organic synthesis, tertiary butyl chloride, proceeds. The first stage is slow, it is associated with the gradual dissociation of a carbonium cation and a chloride anion into a cation. The second stage is faster, the reaction of the carbonium ion with water proceeds. The equation for the substitution of a halogen in an alkane for an oxy group and the preparation of a primary alcohol: (CH 3 ) 3 C — Cl → (CH 3 ) 3 C + + Cl - ; (CH 3 ) 3 C + + H 2 O → (CH 3 ) 3 C — OH + H + . One-stage hydrolysis of primary and secondary alkyl halides is characterized by the simultaneous destruction of the bond of carbon with halogen and the formation of a C – OH pair. This is the mechanism of nucleophilic bimolecular substitution (SN2).
Heterolytic substitution mechanism
The substitution mechanism is associated with electron transfer, the creation of intermediate complexes. The reaction proceeds the faster, the easier it is for the intermediate products characteristic of it to arise. Often, the process goes simultaneously in several directions. The advantage is usually given to the way in which particles are used that require the lowest energy costs for their formation. For example, the presence of a double bond increases the likelihood of the appearance of the allylic cation CH2 = CH — CH 2 + , compared with the ion CH 3 + . The reason lies in the electron density of the multiple bond, which affects the delocalization of the positive charge dispersed throughout the molecule.
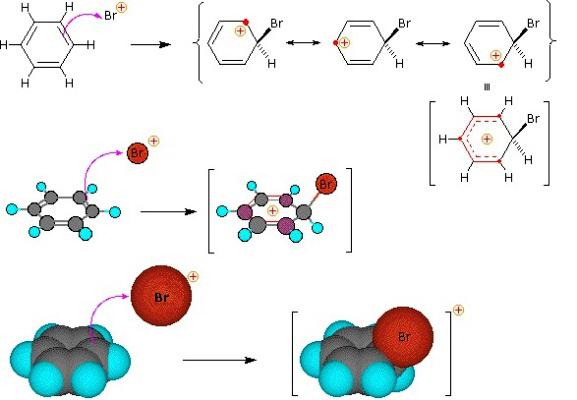
Benzene Substitution Reactions
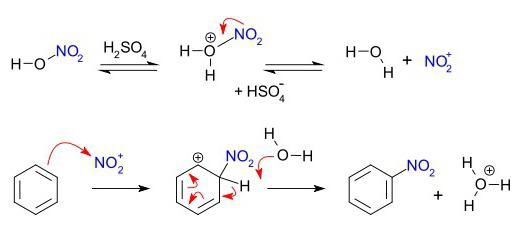
A group of organic compounds that are characterized by electrophilic substitution are arenas. The benzene ring is a convenient object for electrophilic attack. The process begins with polarization of the bond in the second reagent, resulting in the formation of an electrophile adjacent to the electron cloud of the benzene ring. As a result, a transition complex appears. There is still no full-fledged connection between the electrophilic particle and one of the carbon atoms; it is attracted to the entire negative charge of the "aromatic six" electrons. At the third stage of the process, an electrophile and one carbon atom of the ring binds a common pair of electrons (covalent bond). But in this case, the destruction of the "aromatic six" occurs, which is disadvantageous in terms of achieving a stable stable energy state. There is a phenomenon that can be called "proton emission." H + cleavage occurs, and a stable communication system characteristic of arenas is restored. The by-product contains a hydrogen cation from the benzene ring and an anion from the second reagent.
Examples of substitution reactions from organic chemistry
For alkanes, a substitution reaction is especially characteristic. Examples of electrophilic and nucleophilic transformations can be given for cycloalkanes and arenes. Similar reactions in molecules of organic substances occur under ordinary conditions, but more often when heated and in the presence of catalysts. Common and well-studied processes include electrophilic substitution in the aromatic nucleus. The most important reactions of this type:
- Nitration of benzene with nitric acid in the presence of H 2 SO 4 - proceeds according to the scheme: C 6 H 6 → C 6 H 5 —NO 2 .
- Catalytic halogenation of benzene, in particular chlorination, according to the equation: C 6 H 6 + Cl 2 → C 6 H 5 Cl + HCl.
- Aromatic sulfonation of benzene proceeds with "fuming" sulfuric acid, benzenesulfonic acids are formed.
- Alkylation is the replacement of a hydrogen atom from a benzene ring by alkyl.
- Acylation - the formation of ketones.
- Formylation is the replacement of hydrogen by a group of CHO and the formation of aldehydes.
Substitution reactions include the reaction in alkanes and cycloalkanes in which halogens attack the available C — H bond. Derivation can be associated with the substitution of one, two or all hydrogen atoms in saturated hydrocarbons and cycloparaffins. Many of the low molecular weight haloalkanes are used in the production of more complex substances belonging to different classes. The successes in studying the mechanisms of substitution reactions gave a powerful impetus to the development of syntheses based on alkanes, cycloparaffins, arenes, and halogenated hydrocarbons.