Gibbs free energy is a quantity that shows the level of energy change during a chemical reaction, and as a result gives an answer to the question about the possibility of chemical reactions. Such a potential can be taken as the total chemical energy of the system (liquid, crystal, etc.). Gibbs free energy is widely used in chemistry and thermodynamics.
The spontaneous course of isobaric-isothermal processes is determined by the following factors: enthalpy and entropy. The first is associated with a decrease in the enthalpy of the system, and the second is due to an increase in the level of disorder inside the system due to an increase in its entropy. The difference between the described thermodynamic factors is a function of the states of the system, which is known as the isobaric-isothermal potential, or Gibbs free energy (G, kJ).
The spontaneity of the process in an open and closed type system is described by a special criterion, called the Gibbs potential. In fact, it is a state function. D.W. Gibbs, when he worked with thermodynamic systems, derived this function through enthalpy and entropy. Gibbs free energy allows us to predict the direction of the spontaneous biological process, as well as evaluate its theoretically achievable efficiency.
With respect to the second law of thermodynamics, Gibbs conclusions can be formulated as follows: at constant pressure and temperature values without external influence, the system will maintain the level of spontaneous flow only for processes that will result in the Gibbs potential decreasing to the level that will occur when it reaches a steady minimum. So, the equilibrium of the thermodynamic system determines the immutability of free energy. Therefore, the Gibbs potential is a free enthalpy in an isobaric-isothermal system. Let us explain why the minimum is indicated. This is explained by the most important equilibrium postulate in thermodynamics, namely: this condition under the condition of constant pressure and temperature means that for the next change it is necessary to increase the energy level, and this is possible only when external factors change.
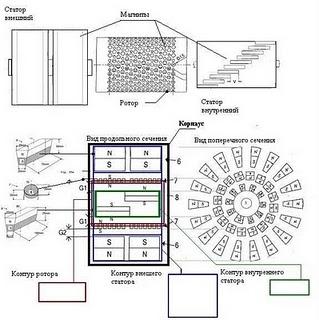
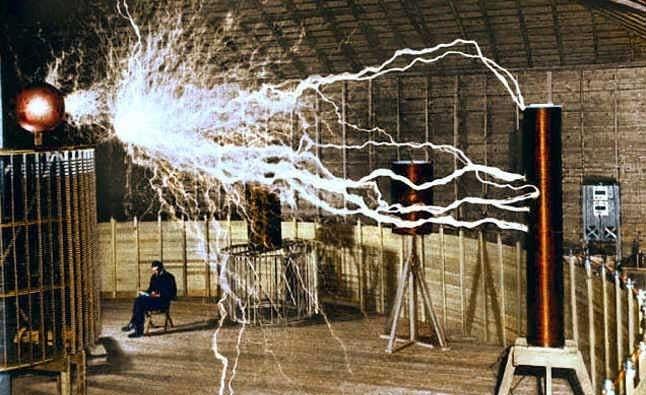
But what is meant by free energy? By this term is meant the process of producing an unlimited amount of energy without or with little energy. That is, the energy received from the solar battery, hydroelectric power station, wind generator is free energy, because we did not spend energy on the fact that the sun's rays fell on the ground, water flowed in the river or the wind blew. There are a lot of similar sources around us, most of them are still unknown to science. Here, from time to time, various inventor experimenters “come across” them. One of these inventions was Tesla’s free energy. According to the scientist, the energy that he received originated from the ether (vacuum). It is a pity that his invention was never brought to its logical conclusion. However, such discoveries continue to be made; this process cannot be stopped. Today, there are many patents for inventions, the basis of which is free energy. A diagram of one of these devices is given above.