Being a unit of living matter, functioning as a complex of open biosystems, the cell constantly exchanges substances and energy with the environment. To maintain homeostasis in it there is a group of special substances of a protein nature - enzymes. The structure, functions, and regulation of enzyme activity are studied by a special branch of biochemistry called enzymology. In this article, using specific examples, we will consider various mechanisms and methods for regulating the activity of enzymes inherent in higher mammals and humans.
Conditions necessary for optimal enzyme activity
Biologically active substances that selectively affect both assimilation reactions and cleavage exhibit their catalytic properties in cells under certain conditions. For example, it is important to find out in which part of the cell a chemical process takes place in which enzymes are involved. Due to compartmentalization (division of the cytoplasm into sections), antagonistic reactions occur in its various parts and organoids.
Thus, protein synthesis is carried out in ribosomes, and their cleavage is carried out in the hyaloplasm. Cellular regulation of the activity of enzymes catalyzing opposite biochemical reactions provides not only the optimal metabolic rate, but also prevents the formation of energetically useless metabolic pathways.
Multienzyme complex
The structural and functional organization of enzymes forms the enzymatic apparatus of the cell. Most of the chemical reactions taking place in it are interconnected. If in a multi-stage chemical process the product of the first reaction is a reagent for the subsequent one, in this case the spatial arrangement of enzymes in the cell is especially pronounced.
It must be remembered that enzymes are by nature simple or complex proteins. And their sensitivity to the cellular substrate is due primarily to a change in the intrinsic spatial configuration of the tertiary or quaternary structure of the peptide. Enzymes also respond to changes not only within cellular parameters, such as the chemical composition of the hyaloplasm, the concentration of reagents and reaction products, temperature, but also to changes that occur in neighboring cells or in intercellular fluid.
Why is the cell divided into compartments
The rationality and logic of the device of wildlife is simply amazing. This fully applies to the vital manifestations characteristic of the cell. For a chemical scientist, it is clear that multidirectional enzymatic chemical reactions, such as glucose synthesis and glycolysis, cannot occur in the same test tube. How, then, do the opposite reactions occur in the hyaloplasm of one cell, which is the substrate for their conduct? It turns out that the cellular content - the cytosol - in which antagonistic chemical processes take place, is spatially separated and forms isolated loci - compartments. Thanks to them, the metabolic reactions of higher mammals and humans are regulated especially precisely, and metabolic products turn into forms that freely penetrate the partitions of cell areas. Then they restore their original structure. In addition to the cytosol, enzymes are found in organelles: ribosomes, mitochondria, nucleus, lysosomes.
The role of enzymes in energy metabolism
Consider the oxidative decarboxylation of pyruvate. The regulation of the catalytic activity of enzymes in it is well studied by enzymology. This biochemical process takes place in the mitochondria, the two-membrane organelles of eukaryotic cells, and is an intermediate process between the oxygen-free breakdown of glucose and the Krebs cycle. Pyruvate dehydrogenase complex - PDH - contains three enzymes. In higher mammals and humans, its decrease occurs with an increase in the concentration of Acetyl-CoA and NATH, that is, in the case of alternative possibilities for the formation of Acetyl-CoA molecules. If the cell needs an additional portion of energy and requires new acceptor molecules to enhance the reactions of the tricarboxylic acid cycle, then the enzymes are activated.
What is allosteric inhibition?
Regulation of enzyme activity can be carried out by special substances - catalytic inhibitors. They can covalently bind to specific loci of the enzyme, bypassing its active center. This leads to deformation of the spatial structure of the catalyst and automatically entails a decrease in its enzymatic properties. In other words, allosteric regulation of enzyme activity occurs. We also add that this form of catalytic action is inherent in oligomeric enzymes, that is, those whose molecules consist of two or more polymer protein subunits. The PDH complex considered in the previous header contains just three oligomeric enzymes: pyruvate dehydrogenase, dehydrolipoyl dehydrogenase, and hydrolipoyl transacetylase.
Regulatory enzymes
Studies in enzymology have established the fact that the rate of chemical reactions depends on both the concentration and activity of the catalyst. Most often, the metabolic pathways contain the main enzymes that control the rate of reactions in all its sections.
They are called regulatory and usually act on the initial reactions of the complex, and can also participate in the most slowly occurring chemical processes in irreversible reactions, or join the reagents at the branch points of the metabolic pathway.
How is peptide interaction carried out
One of the ways in which the regulation of enzyme activity in a cell occurs is through protein-protein interaction. What are you talking about? The regulatory proteins are attached to the enzyme molecule, as a result of which they are activated. For example, the adenylate cyclase enzyme is located on the inner surface of the cell membrane and can interact with such structures as the hormone receptor, as well as with the peptide located between it and the enzyme. Since the intermediate protein changes its spatial confirmation as a result of the combination of the hormone and the receptor, this method of enhancing the catalytic properties of adenylate cyclase in biochemistry is called “activation due to the addition of regulatory proteins”.
Protomers and their role in biochemistry
This group of substances, otherwise called protein kinases, will accelerate the transfer of the PO 4 3- anion to the hydroxo group of amino acids that are part of the peptide macromolecule. We will consider the regulation of protomer enzyme activity using protein kinase A as an example. Its molecule is a tetramer, consists of two catalytic and two regulatory peptide subunits and does not function as a catalyst until four cAMP molecules attach to the regulatory sites of the protomer. This causes a transformation of the spatial structure of the regulatory proteins, which leads to the release of two activated catalytic protein particles, that is, the dissociation of protomers occurs. If cAMP molecules are separated from the regulatory subunits, the inactive protein kinase complex is again reduced to the tetramer, since the association of catalytic and regulatory peptide particles occurs. Thus, the paths of regulation of enzyme activity considered above ensure their reversible character.
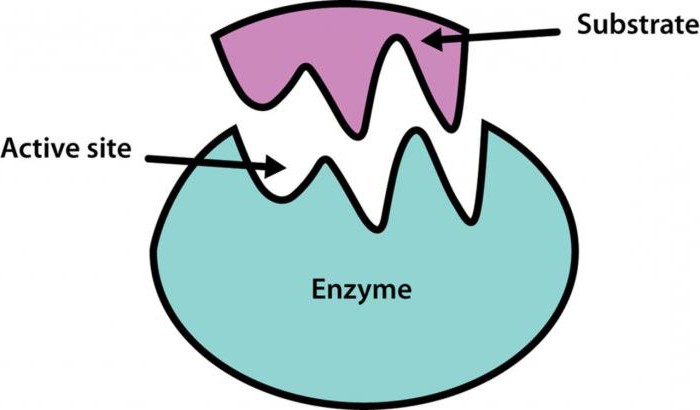
Chemical regulation of enzyme activity
Biochemistry also studied such mechanisms of regulation of enzyme activity as phosphorylation, dephosphorylation. The mechanism of regulation of enzyme activity in this case has the following form: amino acid residues of the enzyme containing OH - groups change their chemical modification due to exposure to phosphoprotein phosphatases. In this case, the active center of the enzyme lends itself to correction , and for some enzymes this is the reason that activates them, and for others, the inhibitory one. In turn, the catalytic properties of phosphoprotein phosphatases themselves are regulated by the hormone. For example, animal starch - glycogen - and fat in the intervals between meals are split in the gastrointestinal tract, more precisely, in the duodenum under the influence of glucagon, a pancreatic enzyme.
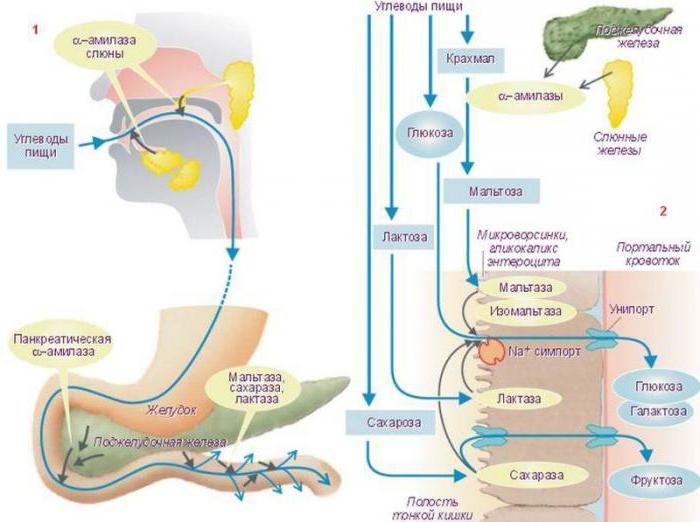
This process is enhanced by phosphorylation of trophic gastrointestinal enzymes. During the period of active digestion, when food enters from the stomach into the duodenum, glucagon synthesis is enhanced. Insulin, another pancreatic enzyme produced by the alpha cells of the islets of Langerhans, interacts with the receptor, including the phosphorylation mechanism of the same digestive enzymes.
Partial proteolysis
As you can see, the levels of regulation of enzyme activity in the cell are diverse. For enzymes located outside the cytosol or organelles (in blood plasma or in the gastrointestinal tract), the process of their hydrolysis of peptide bonds CO-NH serves as a way of their activation. It is necessary, since such enzymes are synthesized in an inactive form. The peptide part is cleaved from the enzyme molecule, and the active center undergoes modification in the remaining structure. This leads to the fact that the enzyme "enters the working state", that is, it becomes able to influence the course of the chemical process. For example, an inactive pancreatic enzyme, trypsinogen, does not break down food proteins that enter the duodenum. In it, under the action of enteropeptidase, proteolysis occurs. After which the enzyme is activated and is now called trypsin. Partial proteolysis is a reversible process. It occurs in cases such as activation of enzymes that break down polypeptides in blood coagulation processes.
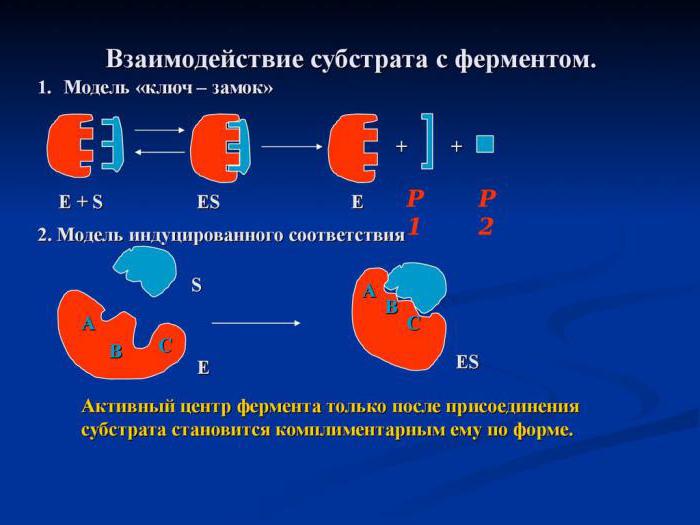
The role of the concentration of starting substances in cell metabolism
The regulation of the activity of the enzyme by the availability of the substrate was partially considered by us in the subtitle “Multienzyme complex”. The rate of catalytic reactions that take place in several stages depends strongly on how many molecules of the starting substance are in the hyaloplasm or organelles of the cell. This is due to the fact that the metabolic path speed is directly proportional to the concentration of the starting substance. The more reagent molecules are in the cytosol, the higher the speed of all subsequent chemical reactions.
Allosteric regulation
Enzymes, the activity of which is controlled not only by the concentration of the starting reagent substances, but also by the effectors, are characterized by the so-called allosteric regulation. Most often, such enzymes are represented by intermediate metabolic products in the cell. Thanks to the effectors, the regulation of enzyme activity is carried out. Biochemistry has proved that such compounds, called allosteric enzymes, are very important for cell metabolism, as they have an extremely high sensitivity to changes in its homeostasis. If an enzyme inhibits a chemical reaction, that is, reduces its speed, it is called a negative effector (inhibitor). In the opposite case, when there is an increase in the reaction rate, we are talking about an activator - a positive effector. In most cases, the starting materials, that is, reagents that enter into chemical interactions, play the role of activators. The final products formed as a result of multi-stage reactions behave as inhibitors. This type of regulation, based on the relationship between the concentration of reagents and products, is called heterotrophic.