In the second half of the nineteenth century, the study of Brownian (chaotic) motion of molecules aroused keen interest among many theoretical physicists of that time. The theory of the molecular-kinetic structure of matter developed by Scottish scientist James Maxwell , although it was generally recognized in European scientific circles, existed only in a hypothetical form. There was no practical confirmation of it then. The motion of the molecules remained inaccessible to direct observation, and the measurement of their speed seemed simply an insoluble scientific problem.
That is why experiments capable of proving in practice the very fact of the molecular structure of matter and determining the speed of motion of its invisible particles were initially perceived as fundamental. The decisive importance of such experiments for physical science was obvious, since it allowed us to obtain practical justification and proof of the validity of one of the most progressive theories of the time - molecular kinetic.
By the beginning of the twentieth century, world science had reached a sufficient level of development for the emergence of real possibilities for experimental verification of Maxwell's theory. The German physicist Otto Stern in 1920, using the molecular beam method, which was invented by the Frenchman Louis Dunoye in 1911, was able to measure the speed of motion of silver gas molecules. Stern's experience irrefutably proved the validity of Maxwell's distribution law. The results of this experiment confirmed the accuracy of the estimate of the average atomic velocities , which stemmed from the hypothetical assumptions made by Maxwell. True, the very nature of high-speed gradation Stern's experience was able to give only very rough information. Science had to wait nine more years for more information.

With greater accuracy, the distribution law was verified by Lammert in 1929, who somewhat improved Stern's experiment by passing a molecular beam through a pair of rotating disks with radial holes and offset relative to each other by a certain angle. By changing the speed of rotation of the unit and the angle between the holes, Lammert was able to isolate individual molecules from the beam that have different speed parameters. But it was Stern’s experience that laid the foundation for experimental research in the field of molecular kinetic theory.

In 1920, the first experimental setup was created necessary for conducting experiments of this kind. It consisted of a pair of cylinders personally designed by Stern. A thin platinum rod with silver coating was placed inside the device, which evaporated when the axis was heated by electricity. Under the vacuum conditions that were created inside the installation, a narrow beam of silver atoms passed through a longitudinal slot cut through the surface of the cylinders and settled on a special external screen. Of course, the aggregate was in motion, and during the time that the atoms reached the surface, it managed to turn around at a certain angle. In this way, Stern determined the speed of their movement.
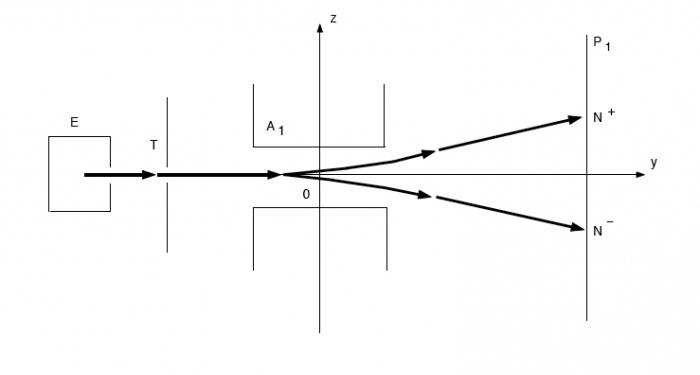
But this is not the only scientific achievement of Otto Stern. A year later, he, together with Walter Gerlach, conducted an experiment that confirmed the presence of spin atoms and proved the fact of their spatial quantization. The Stern – Gerlach experiment required the creation of a special experimental setup with a powerful permanent magnet at its core. Under the influence of the magnetic field generated by this powerful component, elementary particles were deflected according to the orientation of their own magnetic spin.