It is no secret that gasoline is obtained from oil. However, most motorists do not even wonder about how this process of converting oil to fuel for their favorite vehicles occurs. It is called cracking, with its help refineries receive not only gasoline, but also other petrochemical products necessary in modern life. An interesting story is the emergence of this method of oil refining. The Russian scientist is considered the inventor of this process and installation, and the installation itself for this process is very simple and extremely understandable even to a person who does not understand chemistry.
What is cracking?
Why is it called cracking? This word comes from English cracking, meaning splitting. In fact, this is the process of oil refining, as well as its constituent fractions. It is produced in order to obtain products that have a lower molecular weight. These include lubricating oil, motor fuel, and the like. In addition, as a result of this process, the products necessary for the use of the chemical and petrochemical industries are produced.
Alkane cracking involves several processes at once, including condensation and polymerization of substances. The result of these processes is the formation of petroleum coke and a fraction boiling at a very high temperature and called the cracking residue. The boiling point of this substance is more than 350 degrees. It should be noted that, in addition to these processes, there are others - cyclization, isomerization, synthesis.
Shukhov's invention
Oil cracking, its history begins in 1891. Then the engineer Shukhov V.G. and his colleague Gavrilov S.P. invented an industrial plant for continuous thermal cracking. This was the first installation of its kind in the world. Inventors in accordance with the laws of the Russian Empire patented it in the authorized body of their country. Of course, it was an experimental model. Later, after almost a quarter of a century, the technical solutions of Shukhov were the basis for an industrial cracker in the United States. And in the Soviet Union, the first such plants on an industrial scale began to be manufactured and manufactured at the Soviet Cracking Plant in 1934. This factory was located in Baku.
The way the English chemist Barton
At the beginning of the twentieth century, the Englishman Barton, who was engaged in the search for methods and solutions for producing gasoline from oil, made an invaluable contribution to the petrochemical industry. He found an absolutely perfect way, that is, a cracking reaction, as a result of which the greatest amount of light gasoline fractions came out. Prior to this, an English chemist was engaged in the processing of petroleum products, including fuel oil, to extract kerosene. Solving the problem of obtaining gasoline fractions, Barton patented his method of producing gasoline.
In 1916, the Barton method was applied in an industrial environment, and just four years after that, more than eight hundred of its installations were already working at the enterprises.
The well-known dependence of the boiling point of a substance on the pressure on it. That is, if the pressure on a certain liquid is very high, then, accordingly, its boiling temperature will be high. With a decrease in pressure on this substance, it can boil already at a lower temperature. It was this knowledge that the chemist Barton used to achieve the best temperature for the cracking reaction to occur. This temperature is between 425 and 475 degrees. Of course, with such a high temperature effect on oil, it will evaporate, and working with vaporous substances is rather difficult. Therefore, the main task of the English chemist was to prevent boiling and evaporation of oil. He began to conduct the entire process under high pressure.
Cracker
Barton’s device consisted of several elements, including a high-pressure boiler. It was made of fairly thick steel, located above the firebox, which, in turn, was equipped with a smoke pipe. She was directed up to the water collector-cooler. Then this entire pipeline was sent to a tank designed to collect liquid. At the bottom of the tank there was a branched pipe, each tube of which had a control valve.
How was cracking
The cracking process was as follows. The boiler was filled with oil, in particular fuel oil. Gradually, fuel oil was heated by the furnace. When the temperature reached one hundred and thirty degrees, the water in it was removed (evaporated) from the contents of the boiler. Passing through the pipe and cooling, this water fell into the collection tank, and from there it went down the pipe again. At the same time, the process continued in the boiler, during which other components — air and other gases — disappeared from the fuel oil. They went the same way as water, heading into the pipeline.
Having got rid of water and gases, the oil product was ready for subsequent cracking. The furnace was melted more strongly, the temperature of it and the boiler slowly increased until it reached 345 degrees. At this time, light hydrocarbons evaporated. Passing through the pipe to the cooler, they even remained there in a state of gas, unlike water vapor. Once in the collection tank, these hydrocarbons went into the pipeline, as the outlet valve was closed and did not allow them to go into the ditch. They returned through the pipe again to the tank, and then again repeated the whole way, finding no way out.
Accordingly, over time, they became more and more. The result was increasing pressure in the system. When this pressure reached five atmospheres, light hydrocarbons were already unable to evaporate from the boiler. The hydrocarbons contracted to maintain uniform pressure in the boiler, pipeline, collection tank and refrigerator. Due to the high temperature, the decomposition of heavy hydrocarbons began at the same time. As a result, they turned into gasoline, that is, into a light hydrocarbon. Its formation began to occur at about 250 degrees, the light hydrocarbons evaporated upon splitting, and condensation formed in the cooling chamber collected in a collection tank. Further along the pipe, gas flowed into the prepared containers, in which the pressure was reduced. This pressure contributed to the removal of gaseous elements. Over time, such gases were removed, and the finished gas was poured into the desired tanks or tanks.
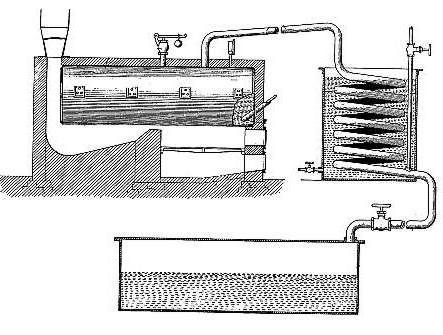
The more light hydrocarbons evaporated, the more elastic and temperature-resistant fuel oil became. Therefore, after the conversion of half the contents of the boiler to gasoline, further work was suspended. The counter installed in the unit helped in determining the amount of gasoline received. The stove was extinguished, the pipeline was blocked. The pipeline valve that connected it to the compressor, on the contrary, opened, the vapor moved into this compressor, the pressure in it was less. In parallel with this, the pipe leading to the obtained gas was blocked to break off its connection with the unit. Further actions were to wait for the boiler to cool, to drain the substance from it. For subsequent use, after that the boiler was cleaned of coke deposits, and a new cracking process could be carried out.
Oil refining stages and Barton installation
It should be noted that the possibility of oil splitting, i.e. cracking alkanes, has long been noticed by scientists. However, it was not used in conventional distillation, since this splitting in this situation was undesirable. For this, superheated steam was involved in the process. With its help, oil was not split, but evaporated.
Over the entire period of its existence, the oil refining industry has gone through several stages. So, from the sixties of the XIX century until the beginning of the last century, oil was refined in order to obtain only kerosene. He was then the material, the substance with which people received lighting in the dark. It is noteworthy that during such processing, light fractions obtained from oil were considered waste. They poured into ditches and destroyed by burning or in another way.
Burton's cracking unit and its method served as a fundamental stage in the entire oil refining industry. It was this method of the English chemist that made it possible to achieve a higher result in producing gasoline. The yield of this refined product, as well as other aromatic hydrocarbons, increased several times.
Cracking requirement
At the beginning of the twentieth century, gasoline was, one might say, an unnecessary oil product. There were very few motor vehicles operating on this type of fuel at that time, and therefore fuel was not in demand. But over time, the fleet of countries grew steadily, respectively, gasoline was also required. In the first ten to twelve years of the twentieth century alone, the need for gasoline increased 115 times!
Obtained by simple distillation of gasoline, or rather, its volumes did not satisfy the consumer, and indeed the manufacturers themselves. Therefore, it was decided to use cracking. This allowed to increase the pace of production. Thanks to this, it was possible to increase the amount of gasoline for the needs of states.
A little later it was found that the cracking of oil products could be carried out not only on fuel oil or diesel fuel. Crude oil was quite suitable for this as feedstock. It was also determined by manufacturers and specialists in this field that the gasoline obtained by the cracking method was of higher quality. In particular, when used in cars, they worked more regularly and longer than usual. This was due to the fact that gasoline obtained by cracking retained some hydrocarbons that burn out during ordinary distillation. These substances, in turn, when used in internal combustion engines, had the property of igniting and burning more smoothly, as a result, the engines worked without fuel explosions.
Catalytic cracking
Cracking is a process that can be divided into two types. It is used to generate fuel, such as gasoline. In some cases, it can be carried out by simple heat treatment of petroleum products - thermal cracking. In other cases, the implementation of this process is possible not only with the help of high temperature, but also with the addition of catalysts. Such a process is called catalytic.
Using the last specified processing method, manufacturers receive high-octane gasoline.
It is believed that this type is the most important process that provides the most deep and high-quality oil refining. The catalytic cracking unit, introduced into the industry in the thirties of the last century, made it possible for manufacturers to obtain undoubted advantages for the entire process. These include operational flexibility, the relative simplicity of combining with other processes (deasphalting, hydrotreating, alkylation, etc.). Thanks to this versatility, a significant share of the use of catalytic cracking in the entire volume of oil refining can be explained.
Raw materials
As a raw material for catalytic cracking, vacuum gas oil is used, which is a fraction having boiling limits from 350 to 500 degrees. In this case, the final boiling point is set differently and directly depends on the metal content. In addition, the cokeability of raw materials also affects this indicator. It can not be more than three tenths of a percent.
Previously required and hydrotreating such a fraction, as a result of which all kinds of sulfur compounds are removed. Hydrotreating also reduces coking properties.
Some well-known companies in the oil refining market have several processes carried out by them, in which cracking of heavy fractions occurs. These include coking oil up to six to eight percent. In addition, hydrocracking residues may be the feedstock. Probably the rarest and, you can say, exotic raw materials are straight-run fuel oil. A similar installation (millisecond technology) is available in the Republic of Belarus at the Mozyr oil refinery.
Literally until recently, when catalytic cracking of petroleum products was used, an amorphous ball catalyst was used. It was a three-five-millimeter balls. Now, cracking catalysts with a volume of not more than 60–80 μm (zeolite-containing microspherical catalyst) are used for this purpose. They consist of a zeolite element located on an aluminosilicate matrix.
Thermal method
As usual, thermal cracking is used to process petroleum products, if you want to get a product with a lower molecular weight. For example, these include unsaturated hydrocarbons, petroleum coke, light motor fuels.
The direction of this oil refining method depends on the molecular weight and nature of the feed, as well as directly on the conditions under which the cracking itself occurs. This has been confirmed by chemists over time. One of the most important conditions that affect the speed and direction of flow of thermal cracking are considered temperature, pressure and duration of the process. The latter receives the visible phase at three hundred to three hundred and fifty degrees. In describing this process, the first-order kinetic cracking equation is used. The result of cracking, or rather, the composition of its products, is affected by a change in pressure. The reason for this is a change in the rate and characteristics of the secondary reactions, which, as mentioned earlier, are the polymerization and condensation that accompany cracking. The equation of the reaction of the thermal process is as follows: 2042 = 1020 + 10 22. The effect on the total and the result is still the volume of reagents.

It should be noted that oil cracking carried out by the above methods is not the only one. In production activities, oil refineries also use many other types of this refining process. So, in certain cases, the so-called oxidative cracking is carried out using oxygen. It is used in production and electric cracking. Using this method, manufacturers produce acetylene by passing methane through electricity.