In order to build a heat engine that can perform work through the use of heat, certain conditions must be created. First of all, the heat engine must operate in a cyclic mode, where a series of sequential thermodynamic processes create a cycle. As a result of the cycle, the gas enclosed in a cylinder with a movable piston does the job. But one cycle for a periodically operating machine is not enough, it must perform cycles over and over again for a certain time. The total work performed during a given time in reality, divided by time, gives another important concept - power.
In the middle of the XIX century, the first heat engines were created. They did the work, but consumed a large amount of heat obtained by burning fuel. It was then that theoretical physicists asked themselves: “How does gas work in a heat engine? How to get maximum work with minimum fuel use? ”
In order to analyze the work of gas , it was necessary to introduce a whole system of definitions and concepts. The combination of all definitions and created a whole scientific direction, called: "Technical thermodynamics." In thermodynamics, a number of assumptions have been adopted that do not detract from the main conclusions. The working fluid is an ephemeral gas (not existing in nature), which can be compressed to zero volume, whose molecules do not interact with each other. In the natural environment, there are only real gases that have well-defined properties that are distinguishable from an ideal gas.
To consider the dynamics models of the working fluid, the laws of thermodynamics were proposed that describe the basic thermodynamic processes, such as:
- isochoric process is a process that is performed without changing the volume of the working fluid. The condition of the isochoric process, v = const;
- isobaric process is a process that is performed without changing the pressure in the working fluid. The condition of the isobaric process, P = const;
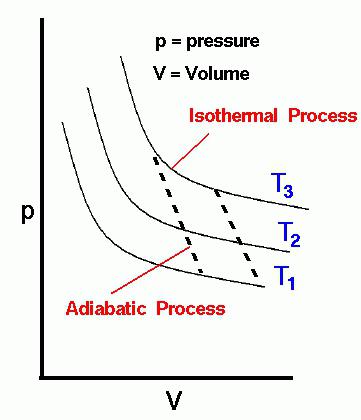
- an isothermal (isothermal) process is a process that is performed while maintaining the temperature at a given level. The condition of the isothermal process, T = const;
- The adiabatic process (adiabatic, as modern heating technicians call it) is a process that takes place in space without exchanging heat with the environment. The condition of the adiabatic process, q = 0;
- polytropic process - this is the most generalized process that describes all of the above-mentioned thermodynamic processes, as well as all the other possible processes in a cylinder with a movable piston.
During the creation of the first heat engines, they were looking for a cycle in which you can get the highest efficiency (efficiency). Sadi Carnot, exploring the totality of thermodynamic processes, on a hunch came to the development of his cycle, which received his name - the Carnot cycle. In it, the isothermal, then adiabatic compression process is sequentially performed. After performing these processes, the working fluid has a supply of internal energy, but the cycle has not yet been completed, therefore, the working fluid expands and performs an isothermal expansion process. To complete the cycle and return to the original parameters of the working fluid, an adiabatic expansion process is performed.
Carnot proved that the efficiency in his cycle reaches a maximum and depends only on the temperatures of two isotherms. The higher the difference between them, the higher the thermal efficiency, respectively. Attempts to create a heat engine according to the Carnot cycle were unsuccessful. This is an ideal cycle that cannot be completed. But he proved the main principle of the second law of thermodynamics about the impossibility of obtaining work equal to the cost of thermal energy. A number of definitions were formulated for the second law of thermodynamics, on the basis of which Rudolf Clausius introduced the concept of entropy. The main conclusion of his research is that entropy is constantly increasing, which leads to thermal "death".
Clausius's most important achievement was an understanding of the essence of the adiabatic process; during its execution, the entropy of the working fluid does not change. Therefore, the adiabatic process according to Clausius is s = const. Here s is entropy, which gives one more name to a process performed without supply or removal of heat, the isoentropic process. The scientist was searching for such a cycle of a heat engine, where there would be no increase in entropy. But, unfortunately, he failed to create this. Therefore, he concluded that a heat engine cannot be created at all.
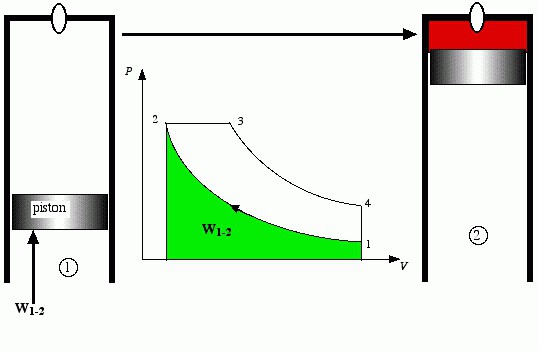
But not all researchers were so pessimistic. They were looking for real cycles for heat engines. As a result of their searches, Nikolaus August Otto created his own cycle of a heat engine, which today is implemented in gasoline powered engines. Here, the adiabatic process of compression of the working fluid and isochoric heat supply (combustion of fuel at a constant volume) are performed, then the expansion adiabat (work is performed by the working fluid in the process of increasing its volume) and isochoric heat removal appear. The first Otto cycle internal combustion engines used combustible gases as fuel. Much later, carburetors were invented, which began to create gasoline-air mixtures of air with gasoline vapors and feed them into the engine cylinder.
In the Otto cycle, the combustible mixture is compressed, therefore, its compression ratio is relatively small - the combustible mixture tends to detonate (explode when critical pressures and temperatures are reached). Therefore, the work in the adiabatic compression process is relatively small. Here another concept is introduced: the compression ratio is the ratio of the total volume to the compression volume.
The search for ways to increase fuel energy efficiency continued. An increase in efficiency is seen in an increase in the compression ratio. Rudolf Diesel developed his own cycle, in which heat is supplied at constant pressure (in the isobaric process). Its cycle formed the basis of engines using diesel fuel (it is also called diesel fuel). In a diesel cycle, not a combustible mixture is compressed, but air. Therefore, they say that work is being done in the adiabatic process. The temperature and pressure at the end of compression are high, so fuel is injected through the nozzles. It mixes with hot air, forms a combustible mixture. It burns, while the internal energy of the working fluid increases. Further, gas expansion proceeds along the adiabat, and a working stroke is made.
An attempt to realize the Diesel cycle in heat engines failed, so Gustav Trinkler created a combined Trinkler cycle. It is used in today's diesel engines. In the Trinkler cycle, heat is supplied through the isochore and then through the isobar. Only after this is the adiabatic process of expansion of the working fluid.
By analogy with piston heat engines, turbine ones also work. But in them, the process of heat removal at the end of the useful adiabatic expansion of the gas is carried out along the isobar. On airplanes with gas turbine and turboprop engines, the adiabatic process takes place twice: during compression and expansion.
To substantiate all the fundamental concepts of the adiabatic process, calculation formulas were proposed. An important quantity appears here, called the adiabatic index. Its value for a diatomic gas (oxygen and nitrogen are the main diatomic gases present in the air) is 1.4. Two more interesting characteristics are used to calculate the adiabatic index, namely: isobaric and isochoric heat capacities of the working fluid. Their ratio k = Cp / Cv is the adiabatic exponent.
Why is the adiabatic process used in theoretical cycles of heat engines? In fact, polytropic processes are carried out, but due to the fact that they occur at a high speed, it is customary to assume the absence of heat exchange with the environment.
90% of the electricity is generated in thermal power plants. They use water vapor as a working fluid. It is obtained by boiling water. To increase the working potential of the steam, it is overheated. Then, at high pressure, superheated steam is supplied to the steam turbine. Here, the adiabatic process of expansion of steam also takes place. The turbine receives rotation, it is transmitted to the generator. He, in turn, generates electricity for consumers. Steam turbines operate on the Rankine cycle. Ideally, an increase in efficiency is also associated with an increase in temperature and pressure of water vapor.
As can be seen from the above, the adiabatic process is very common in the production of mechanical and electrical energies.